Risedronate Sodium |
| رقم الكتالوجGC14110 |
Risedronate sodium هو بيريدينيل ثنائي الفوسفونات الذي يثبط ارتشاف العظم بوساطة ناقضات العظم
Products are for research use only. Not for human use. We do not sell to patients.
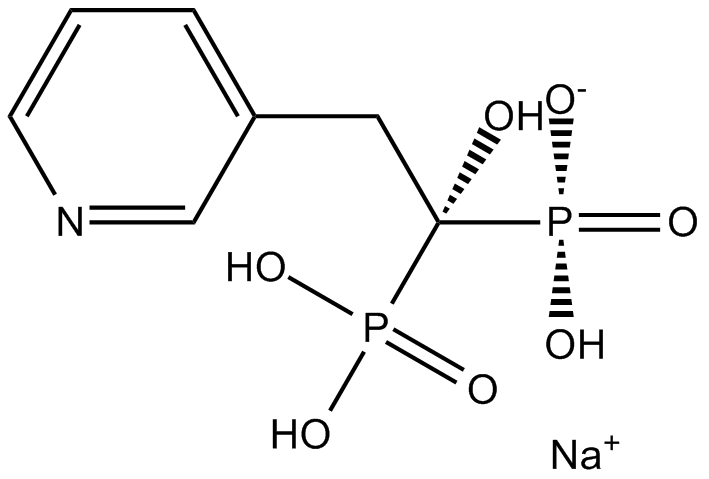
Cas No.: 115436-72-1
Sample solution is provided at 25 µL, 10mM.
Risedronate sodium is a pyridinyl biphosphonate which inhibits osteoclast-mediated bone resorption.Target: Risedronate sodium, which was promoted in Croatia a few months ago, is the latest (III) generation of bisphosphonates, the most efficient anti-resorption drugs that inhibit osteoclast-mediated bone resorption and change the bone metabolism. Risedronate sodium is hence the first line of bisphosphonates for the reduction of vertebral and non-vertebral fracture risks in postmenopausal women with osteoporosis or those with a high risk of osteoporosis. It also efficiently prevents bone loss or improves bone density in men and women on a long-term corticosteroid therapy [1].The administration of 20 and 25 mg/kg risedronate sodium for 4 days led to decreases of parasitemia of 68.9% and 83.6%, respectively. On the seventh day of treatment the inhibitions were 63% and 88.9% with 20 and 25 mg/kg, respectively. After recovering the parasitemia, a dose-response curve was obtained for estimating the ID50 (dose causing 50% inhibition), equivalent to 17 ± 1.8 mg/kg after 7 days of treatment. Four days after the interruption of treatment (11 days postinfection), the parasitemias of the groups treated with 10, 15, 20, and 25 mg/kg/day were 15.3%, 15.9%, 15.2%, and 5.7%, respectively. Conversely, the group that received PBS presented parasitemia of 25.6%. Among the groups treated with risedronate sodium, only the animals that received 25 mg/kg had a significant inhibition of 77.8% (see Table S1 in the supplemental material), demonstrating that even after treatment discontinuation, the parasitemia of the animals remained low in relation to that of the controls [2].Clinical indications: Bone resorption; Male osteoporosis; Osteogenesis imperfecta; Osteoporosis; Pagets bone disease Toxicity: abdominal pain; anxiety, back pain; belching, bladder irritation; bone disorders and pain; bronchitis; bursitis; cataracts; chest pain; colitis; constipation; depression; diarrhea; difficulty breathing
References:
[1]. Giljevic Z, et al. Treatment of osteoporosis by risedronate-- speed, efficacy and safety. Reumatizam. 2006;53(2):66-71.
[2]. Jordao FM, et al. In vitro and in vivo antiplasmodial activities of risedronate and its interference with protein prenylation in Plasmodium falciparum. Antimicrob Agents Chemother. 2011 May;55(5):2026-31.
Average Rating: 5 (Based on Reviews and 1 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















