Actinomycin D (Synonyms: Cosmegen, Dactinomycin, Meractinomycin, NCI C04682, NSC 3053, Oncostatin K) |
| رقم الكتالوجGC16866 |
مانع تفاعل الحمض النووي مع المتكلمات الجينية ذو التأثير المضاد للسرطان
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 50-76-0
Sample solution is provided at 25 µL, 10mM.
- Mol Cancer 20.1 (2021): 1-17.PMID:34852843
- J Biomed Res 35.6 (2021): 411.PMID:34857678
- Reprod Sci 29.8 (2022): 2414-2427.PMID:34981461
- J Exp Clin Canc Res 41.1 (2022): 33.PMID:35073964
- Cell Chem Biol 29.7 (2022): 1218-1231.PMID:35245437
- Thorac Cancer 13.9 (2022): 1299-1310.PMID:35411716
- Front Chem 10 (2022): 911201.PMID:35755263
- Cell Mol Biol Lett1 27.1 (2022): 51.PMID:35761192
- Cell Death Dis 13.8 (2022): 715.PMID:35977935
- Histol Histopathol 35.9 (2020): 919-927.PMID:32282924
- Cancers 14.21 (2022)5218.PMID:36358640
- J cell physiol 238.1 (2023): 195-209.PMID:36436184
- Appl biol chem 65.1 (2022): 81.
- Pathol Res Pract 243 (2023): 154317.PMID:36738516
- Apoptosis (2023): 1-11.PMID:36645573
- Pharmacol Res 189 (2023): 106700.PMID:36796466
- Environ Pollut 325 (2023): 121393.PMID:36878272
- Int Heart J 64.3 (2023): 442-452.PMID:37258120
- Hepatol Commun 7.7 (2023).PMID:37314767
- Acta Pharm Sin B 13.2 (2023): 598-617.PMID:36873185
- Cell Death Discov 8.1 (2022): 279.PMID:35676262
- Cell Mol Biol Lett 27.1 (2022): 51.PMID:35761192
- Front Immunol 13 (2023): 1094556.PMID:36685533
- Cell Death Discov 9.1 (2023): 219.PMID:37393317
- iScience 25.7 (2022).PMID:35811843
- Pathol Res Pract 243 (2023): 154317.PMID:36738516
- Environ Health Persp 131.9 (2023): 097004.PMID:37682722
- Adv Sci(2023): 2303113.PMID:37877615
- Oncogene (2023): 1-13.PMID:38040806
- J Neuroimmunol (2023):578265.
- J Cell Mol Med (2023).PMID:38146129
- Cell Commun Signal 22.1 (2024):1-14.PMID:38233839
- Cell Death Dis 15.1 (2024):97.PMID:38286802
- Cell Biol Toxicol 40.1 (2024):1-18.
- Cell Commun Signal 22.1 (2024):51.PMID:38233839
- Sci Total Environ (2024):170701.PMID:38325452
- Andrology-Us 12.3 (2024):643-654.PMID:37644905
- Cell Death Dis 15.2 (2024):171.PMID:38402183
- Histol Histopathol (2024):18720-18720.PMID:38390782
- Hum Mol Genet (2024):ddae040.PMID:38491801
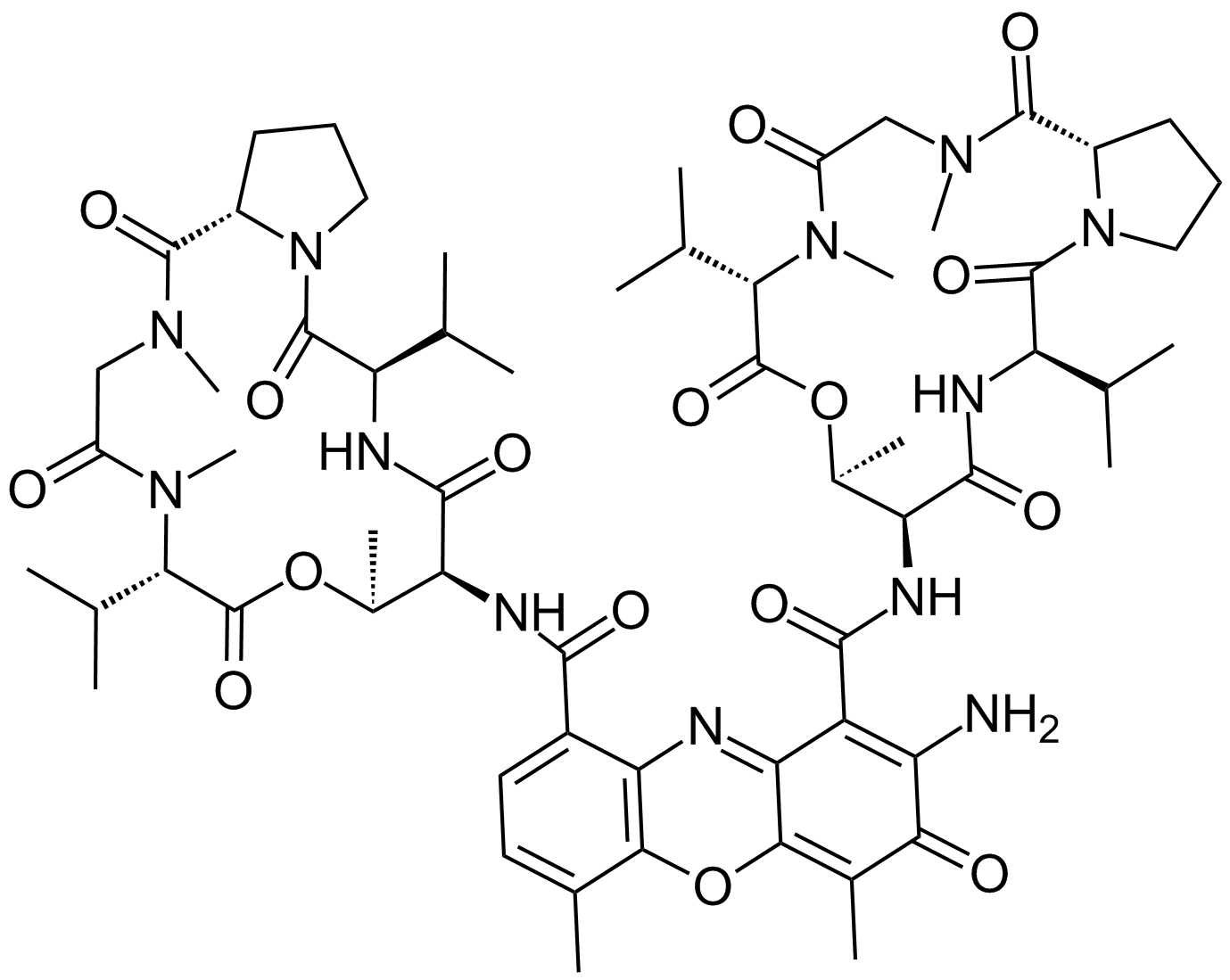
Actinomycin D (dactinomycin) is a natural chromopeptide isolated from Streptomyces species, and has one heterocyclic chromophore and two cyclic pentapeptide lactone rings. [1] It is the first antibiotic showing anti-tumor activity, and has been implemented in the clinical practice for years to treat, such as testicular cancer, and choriocarcinoma.[2]
Actinomycin D intercalates into DNA to inhibit the transcription. It forms a very stable complex with DNA, preventing the unwinding of the DNA double-helix, so as to inhibit the DNA-dependent RNA polymerase activity. Actinomycin D is well implemented in mRNA stability assays to inhibit the synthesis of new mRNA, allowing the assessment of mRNA decay by measuring mRNA abundance following transcription inhibition. [3]
The in vitro experiment suggests that actinomycin D is an potent and effective agent to inhibit the proliferation of SMC by preventing cells from getting into S phase. The LD50 (260 lM) determined by measuring the remaining viable cells at various concentrations of actinomycin D was about five orders greater than that of IC50 (0.4 nM), which was calculated by measuring the percentage of cells in S phase following the treatment of actinomycin D. A dose-dependent inhibition by actinomycin D was found in PCNA, Raf and FAK. However, in contrast to those seen on PCNA, Raf and FAK expression, the phosphorylated Erk was significantly up-regulated by actinomycin D. An in vivo study using rat carotid artery as a model was conducted to evaluate if topically applied actinomycin D onto the arterial adventitia of the artery was effective in suppressing the formation of stenosis following a balloon angioplasty. Topical application of pluronic gel containing 80 nM and 80 μM actinomycin D to surround the adventitia of rat carotid arteries, the thickness of the neointima was significantly reduced (45% and 55%, respectively). [4]
Reference:
[1]. Farber S. Chemotherapy in the treatment of leukemia and Wilms' tumor. JAMA. 1966 Nov 21;198(8):826-36. PMID: 4288581.
[2]. Lewis J.L., Jr. Chemotherapy of gestational choriocarcinoma. Obstet. Gynecol. Surv. 1973;28:7478–7480. doi: 10.1097/00006254-197307000-00006.
[3]. Shyu A. B., Greenberg M. E. and Belasco J. G.(1989). The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev 3(1): 60-72.
[4]. Wu, C. H., Pan, J. S., Chang, W. C., Hung, J. S., & Mao, S. J. T. (2005). The molecular mechanism of actinomycin D in preventing neointimal formation in rat carotid arteries after balloon injury. Journal of Biomedical Science, 12(3), 503–512. doi:10.1007/s11373-005-6900-5.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




