UVI 3003 |
| رقم الكتالوجGC13199 |
UVI 3003 هو مضاد انتقائي للغاية لمستقبلات الريتينويد X (RXR) ، ويمنع Xenopus و RXRα البشري في خلايا Cos7 ، مع IC50s من 0.22 و 0.24 ميكرومتر ، على التوالي
Products are for research use only. Not for human use. We do not sell to patients.
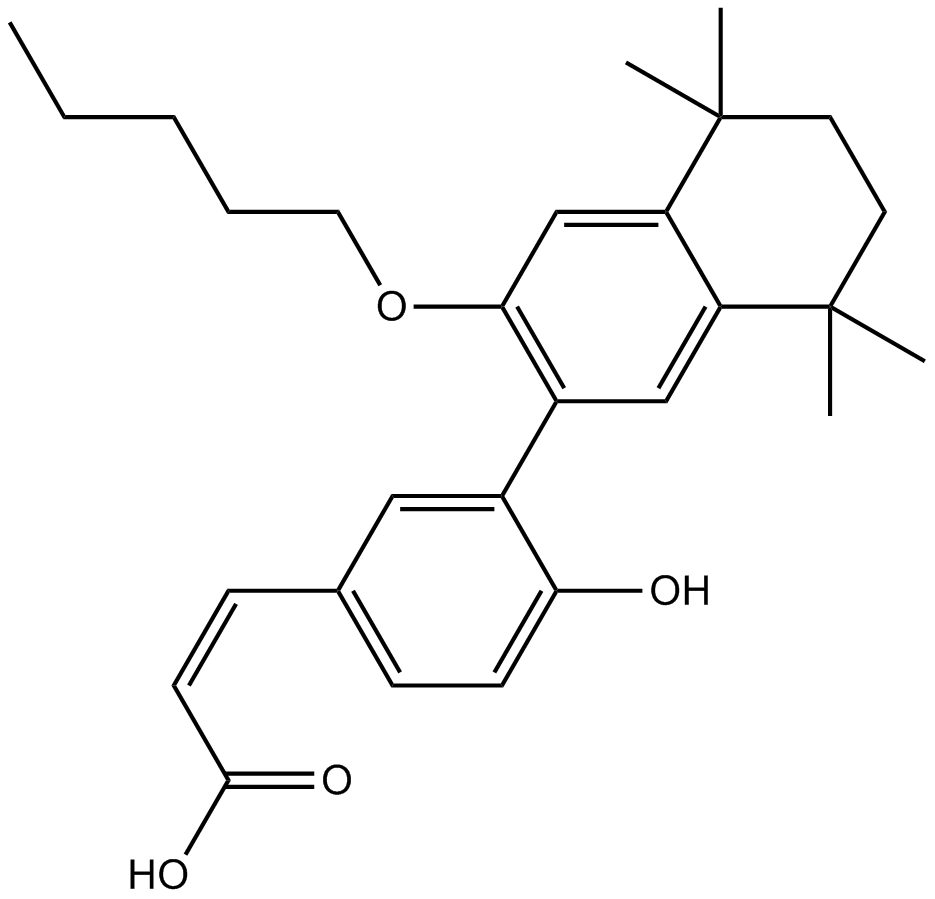
Cas No.: 847239-17-2
Sample solution is provided at 25 µL, 10mM.
UVI 3003 is a highly selective antagonist of retinoid X receptor (RXR), and inhibits xenopus and human RXRα in Cos7 cells, with IC50s of 0.22 and 0.24 μM, respectively.
UVI3003 inhibits the activity of xenopus and human RXRα, with IC50s of 0.22 and 0.24 μM, respectively. UVI3003 fully activates xPPARγ with an EC50 of 12.6 μM, and is almost completely inactive on hPPARγ and mPPARγ[1]. UVI 3003 (10 μM) does not change the proliferation rate of extraocular muscles (EOM)-derived or LEG-derived EECD34 cells. UVI 3003 causes a 65.4% difference in EECD34 cell fusion and desmin expression[2].
References:
[1]. Zhu J, et al. The unexpected teratogenicity of RXR antagonist UVI3003 via activation of PPARγ in Xenopus tropicalis. Toxicol Appl Pharmacol. 2017 Jan 1;314:91-97.
[2]. Hebert SL, et al. Effects of retinoic acid signaling on extraocular muscle myogenic precursor cells in vitro. Exp Cell Res. 2017 Dec 1;361(1):101-111.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















