Epelsiban (GSK 557296) (Synonyms: GSK 557296) |
| رقم الكتالوجGC30594 |
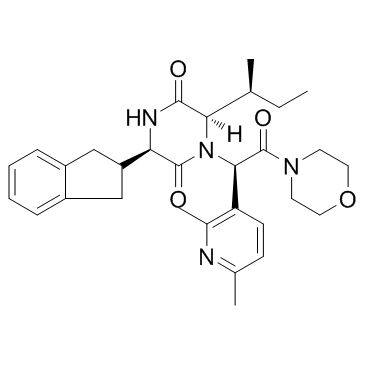
Epelsiban (GSK 557296) (GSK 557296) هو مضاد لمستقبلات الأوكسيتوسين قوي وانتقائي ومتوفر بيولوجيًا عن طريق الفم ، مع pKi يبلغ 9.9 لمستقبلات الأوكسيتوسين البشري.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 872599-83-2
Sample solution is provided at 25 µL, 10mM.
Epelsiban (GSK 557296) is a potent, selective and orally bioavailable oxytocin receptor antagonist, with a pKi of 9.9 for human oxytocin receptor.
Epelsiban is a potent oxytocin receptor, with a pKi of 9.9 for human oxytocin receptor, >31000-fold selectivity over all three human vasopressin receptors hV1aR (pKi,
Epelsiban shows an IC50 of 192 nM for oxytocin receptor in rats. Epelsiban has low levels of intrinsic clearance against the microsomes of rat, dog, and cynomolgus monkey, good bioavailability (55%), but is negative in the genotoxicity screens with a satisfactory oral safety profile in female rats[1].
[1]. Borthwick AD, et al. Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: synthesis, pharmacokinetics, and in vivo potency. J Med Chem. 2012 Jan 26;55(2):783-96.
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















