Lemborexant (Synonyms: E-2006) |
| رقم الكتالوجGC19220 |
Lemborexant (E-2006) هو مضاد مزدوج عكسي وتنافسي ونشط فمويًا لمستقبلات orexin OX1 و OX2 بقيم IC50 تبلغ 6.1 نانومتر و 2.6 نانومتر ، على التوالي
Products are for research use only. Not for human use. We do not sell to patients.
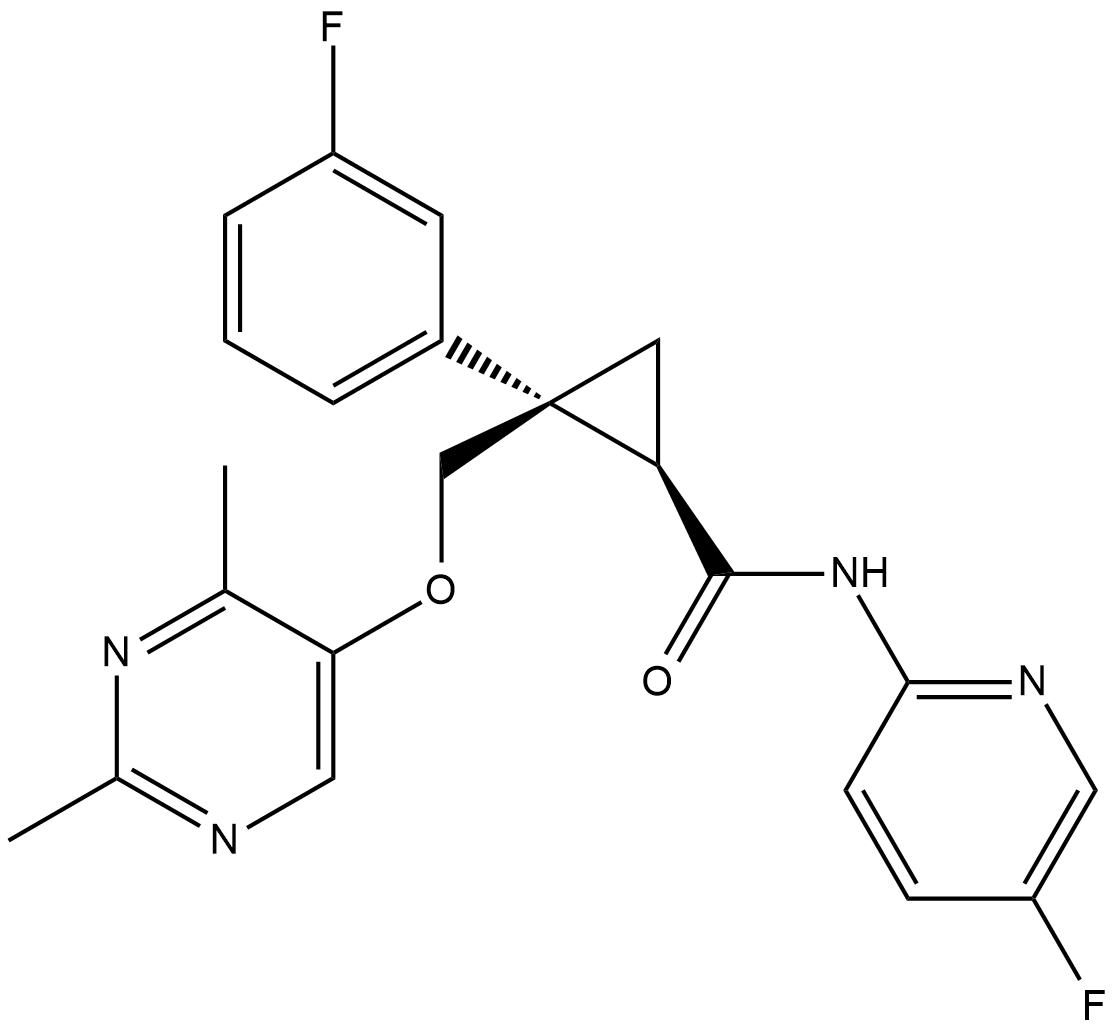
Cas No.: 1369764-02-2
Sample solution is provided at 25 µL, 10mM.
Lemborexant (E2006) is an oral active diorexin receptor antagonist (DORA) and has been approved by the US Food and Drug Administration for the treatment of insomnia[1-2].
Lemborexant(1-25mg;15days) had a positive effect on Sleep Efficiency (SE) by improving both sleep onset (decreasing LPS) and sleep maintenance (decreasing WASO) in a dose-related manner[2]. In 62 subjects, according to circadian rhythm, day and night parameters (placebo, n = 12; lemborexant 2.5 mg [LEM2.5], n = 12; lemborexant 5 mg [LEM5], n = 13, lemborexant 10 mg [LEM10], n = 13 and lemborexant 15 mg [LEM15], n = 12). Mean least active 5 hours (L5) showed a decrease from baseline to week 4 for LEM2.5, LEM5 and LEM15 that was significantly greater than with placebo, suggesting a reduction in restlessness[3].
References:
[1]. Dayvigo [prescribing information] (lemborexant), Woodcliff Lake, NJ: Eisai Inc. US. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212028s000lbl.pdf. Accessed January 08, 2020.
[2]. Murphy P, Moline M, et,al. Lemborexant, A Dual Orexin Receptor Antagonist (DORA) for the Treatment of Insomnia Disorder: Results From a Bayesian, Adaptive, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Sleep Med. 2017 Nov 15;13(11):1289-1299. doi: 10.5664/jcsm.6800. PMID: 29065953; PMCID: PMC5656478.
[3]. Moline M, Thein S, et,al. Safety and Efficacy of Lemborexant in Patients With Irregular Sleep-Wake Rhythm Disorder and Alzheimer's Disease Dementia: Results From a Phase 2 Randomized Clinical Trial. J Prev Alzheimers Dis. 2021;8(1):7-18. doi: 10.14283/jpad.2020.69. PMID: 33336219.
Average Rating: 5 (Based on Reviews and 16 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















