Linezolid (Synonyms: PNU 100766) |
| رقم الكتالوجGC11221 |
Linezolid (PNU-100766) هو أول عضو في فئة المضادات الحيوية الاصطناعية Oxazolidinone.
Products are for research use only. Not for human use. We do not sell to patients.
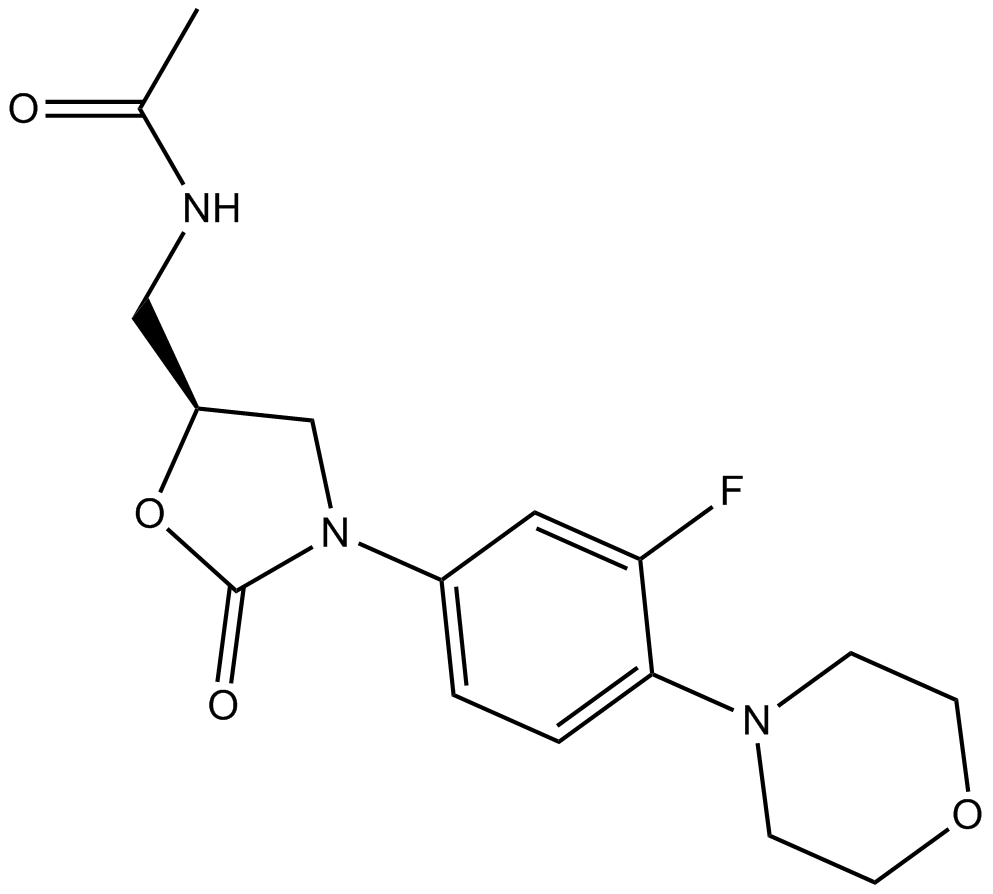
Cas No.: 165800-03-3
Sample solution is provided at 25 µL, 10mM.
Linezolid, a synthetic oxazolidinone antimicrobial, shows a wide spectrum against Gram-positive bacteria andmultidrug-resistant bacteria such as anaerobes, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci, penicillin-resistant pneumococci and streptococcus [1,2].
Oxazolidinones could inhibit protein synthesis by binding to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevent the formation of a functional 70S-initiation complex. Linezolid is also a weak, nonselective and reversible inhibitor of monoamine oxidase [2].
In Vitro: Linezolid was a potent inhibitor of cell-free transcription-translation in E. coli. IC50 was 1.8 mM[3]. linezolid MICs vary slightly owing to the different test method and laboratory. The MIC values were between 0.5 and 4 mg/L for streptococci, enterococci and staphylococci [4].
Clinical Trials: Linezolid is fully bioavailable following oral administration, with maximum plasma linezolid concentrations achieved between 1 and 2 hours after oral administration. The elimination half-life of linezolid is 5–7 hours, and twice-daily administration of 400–600mg provides steady-state concentrations in the therapeutic range[5].
In clinical trials involving hospitalised patients with skin/soft tissue infections (predominantly S. aureus), intravenous/oral administration of linezolid (up to 1250 mg/day) produced clinical success in > 83% of individuals. In patients with community-acquired pneumonia, success rates were > 94%[6]. Linezolid could also be used in patients with nosocomial pneumonia[7].Linezolid appears to be well tolerated and the most common side effects is gastrointestinal disturbances [6].
References:
[1] Tsiodras S, Gold H S, Sakoulas G, et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus[J]. The Lancet, 2001, 358(9277): 207-208.
[2] Swaney S M, Aoki H, Ganoza M C, et al. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria[J]. Antimicrobial agents and chemotherapy, 1998, 42(12): 3251-3255.
[3] Shinabarger D L, Marotti K R, Murray R W, et al. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions[J]. Antimicrobial agents and chemotherapy, 1997, 41(10): 2132-2136.
[4] Livermore D M. Linezolid in vitro: mechanism and antibacterial spectrum[J]. Journal of antimicrobial chemotherapy, 2003, 51(suppl 2): ii9-ii16.
[5] Stalker D J, Jungbluth G L. Clinical pharmacokinetics of linezolid, a novel oxazolidinoneantibacterial[J]. Clinical pharmacokinetics, 2003, 42(13): 1129-1140.
[6] Clemett D, Markham A. Linezolid[J]. Drugs, 2000, 59(4): 815-827.
[7] Wunderink R G, Cammarata S K, Oliphant T H, et al. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia[J]. Clinical therapeutics, 2003, 25(3): 980-992.
Average Rating: 5 (Based on Reviews and 33 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















