Cyclooxygenase
منتجات لـ نبسب؛ Cyclooxygenase
- القط. رقم اسم المنتج بيانات
-
GC41431
δ12-Prostaglandin D2
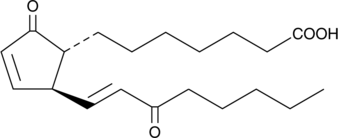
Δ12PGD2
Prostaglandin D2 (PGD2) is one of the five primary enzymatic prostaglandins derived directly from PGH2.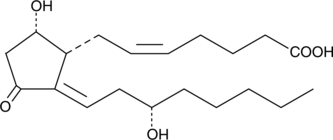
-
GC41109
δ12-Prostaglandin J2
Δ12PGJ2
δ؛ 12-Prostaglandin J2 (δ؛ 12-PGJ2) عبارة عن سيكلوبنتينون بروستاغلاندين (PG) له تأثير مضاد للتكاثر على نمو خلايا الورم المختلفة. δ ؛ 12-Prostaglandin J2 ، وهو منتج طبيعي للجفاف من البروستاغلاندين D2 ، قادر على إحداث موت الخلايا المبرمج في خلايا هيلا عن طريق تنشيط كاسباس.
-
GC41476
δ17-6-keto Prostaglandin F1α
ω3 6keto PGF2α
δ17-6-keto Prostaglandin F1α (δ17-6-keto PGF1α) is a cyclooxygenase (COX) product of eicosapentaenoic acid (EPA) in various tissues such as seminal vesicles, lung, Polymorphonuclear leukocytes, and ocular tissues.
-
GC49515
(±)-Ibuprofen-d3 (sodium salt)
DL-Ibuprofen-d3
An internal standard for the quantification of (±)-ibuprofen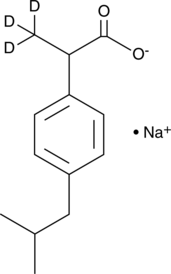
-
GC45278
(±)-Ketoprofen-d3
(R,S)-Ketoprofen-d3, 2-(3-benzoylphenyl)Propionic Acid-d3
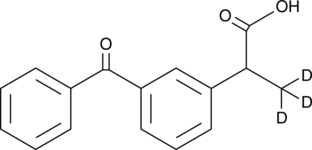
-
GC40270
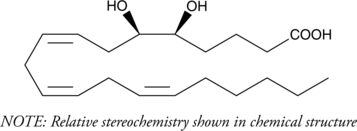
(±)5(6)-DiHET
(±)5,6-DiHETrE
5(6)-DiHET is a fully racemic version of the enantiomeric forms biosynthesized from 5(6)-EET by epoxide hydrolases.
-
GC40272
(±)8(9)-DiHET
(±)8,9DiHETrE
Epoxide hydrolases convert the EETs into vicinal diols, with the concurrent loss of much of their biological activity.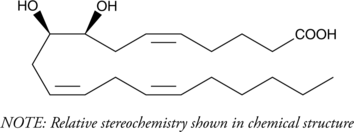
-
GC40281
(+)-15-epi Cloprostenol
DCloprostenol, (+)15(S)Cloprostenol
Cloprostenol is a synthetic prostaglandin F2α (PGF2α) analog and a potent FP receptor agonist.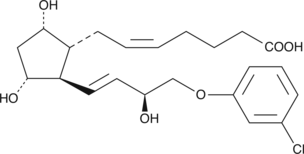
-
GC40282
(+)-5-trans Cloprostenol
DCloprostenol, (+)5,6trans Cloprostenol, (+)5trans16mchlorophenoxy tetranor PGF2α, (+)5trans16mchlorophenoxy tetranor Prostaglandin F2α
Cloprostenol is a synthetic derivative of prostaglandin F2α that is used in veterinary medicine as a luteolytic agent for the induction of estrus and in the treatment of reproductive disorders in cattle, swine, and horses.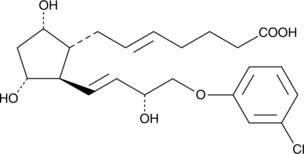
-
GC45260
(+)-Cloprostenol isopropyl ester
(+)-5-cis Cloprostenol isopropyl ester, (+)16mchlorophenoxy tetranor Prostaglandin F2α isopropyl ester
(+) -كلوبروستينول إيزوبروبيل إستر ، بروستاغلاندين F2α ؛ النظير هو وسيط من (+) -كلوبروستينول.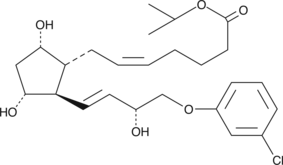
-
GC45262
(+)-Cloprostenol methyl ester
DCloprostenol methyl ester, (+)16mChlorophenoxy tetranor PGF2α methyl ester
(+)-Cloprostenol is a synthetic analog of prostaglandin F2α (PGF2α).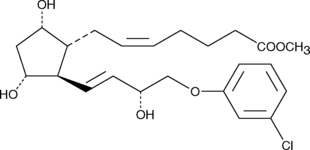
-
GC46245
(-)-G-Lactone
A bicyclic γ-lactone
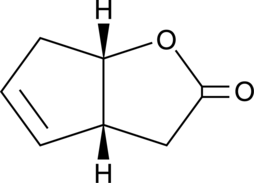
-
GC48508
(4-Carboxybutyl-d4)triphenylphosphonium (bromide)
TPP-d4, 5-Triphenylphosphoniovaleric Acid-d4
An internal standard for the quantification of (4-carboxybutyl)triphenylphosphonium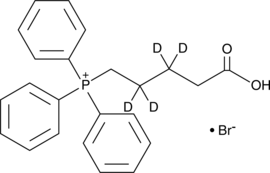
-
GC40716
(R)-Butaprost
(±)15deoxy16Rhydroxy17cyclobutyl PGE1 methyl ester, 15deoxy16Rhydroxy17cyclobutyl PGE1 methyl ester, TR 4978
Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype.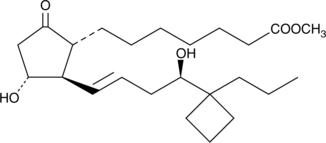
-
GC41714
(R)-Butaprost (free acid)
(±)15deoxy16Rhydroxy17cyclobutyl PGE1, 15deoxy16Rhydroxy17cyclobutyl PGE1
Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype.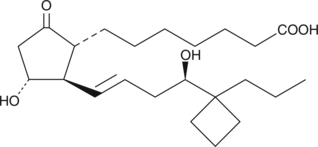
-
GC41723
(S)-(-)-1,1'-Bi-2-naphthol
(S)BINOL
S-BINOL is a chiral auxiliary for asymmetric ketone reduction, in particular 15-keto prostaglandin intermediates to 15(S)-alcohols.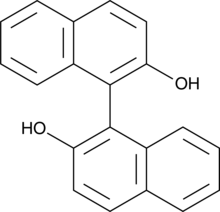
-
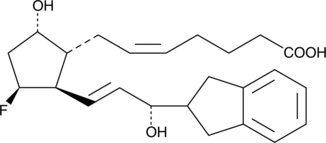
GC41735
(S)-AL 8810
AL 8810 is an 11β-fluoro analog of PGF2α which acts as a potent and selective antagonist at the FP receptor.
-
GC49179
(S)-O-Desmethyl Naproxen
(S)-6-Desmethyl Naproxen
A metabolite of (S)-naproxen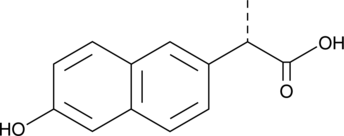
-
GC45948
1,2,3-Trilinoelaidoyl-rac-glycerol
TG(18:2(9E,12E)/18:2(9E,12E)/18:2(9E,12E)), Trilinoelaidin
A triacylglycerol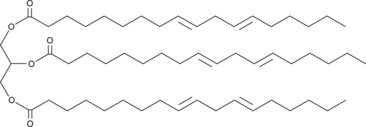
-
GC41761
1-(6-Methoxy-2-naphthyl)ethanol
Naproxen Impurity K
1-(6-Methoxy-2-naphthyl)ethanol is a potential impurity in commercial preparations of naproxen.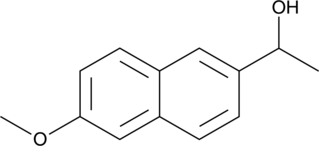
-
GC49366
1-Salicylate Glucuronide
Salicyl Phenolic Glucuronide, Salicylic Acid Phenolic Glucuronide
A metabolite of salicylic acid and aspirin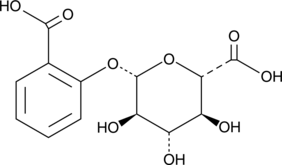
-
GC40577
11β-13,14-dihydro-15-keto Prostaglandin F2α
11β13,14dihydro15keto PGF2α, 11-epi-13,14-dihydro-15-keto PGF2α
11β-13,14-dihydro-15-keto Prostaglandin F2α (11β-13,14-dihydro-15-keto PGF2α) is a metabolite of Prostaglandin D2 (PGD2) in the 15-hydroxy PGDH pathway.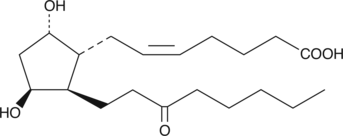
-
GC41243
11β-Misoprostol
Misoprostol is a widely sold analog of prostaglandin E1 (PGE1) which has potent but relatively non-selective agonist activity with respect to the prostanoid EP receptor subgroup.
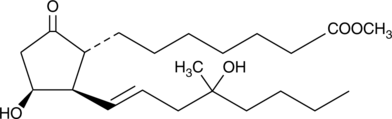
-
GC41410
11β-Prostaglandin E2
11β-PGE2
11β؛ -Prostaglandin E2 (11β؛ -Dinoprostone) ، مشتق Prostanoid ، يمنع [3H] PGE2 الارتباط بالأغشية الوطائية في الفئران مع Ki من 53.3 نانومتر.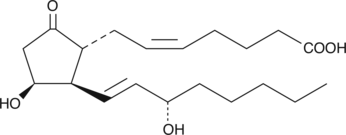
-
GC41432
11β-Prostaglandin F1β
9β,11βPGF1α, 11βPGF1β, 11epi PGF1β
11β-PGF1β is the stereoisomer of PGF1α with both C-9 and C-11 hydroxyls inverted.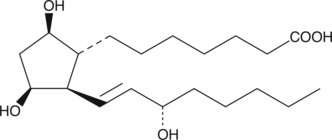
-
GC41404
11β-Prostaglandin F2α Ethanolamide
11-epi PGF2α-EA, 11β-PGF2α-EA, 11β-Prostamide F2α
11β-Prostaglandin F2α ethanolamide (11β-PGF2α-EA) is the theoretical hepatic metabolite of PGD2-EA, produced during COX-2 metabolism of the endogenous cannabinoid AEA which is found in brain, liver, and other mammalian tissues.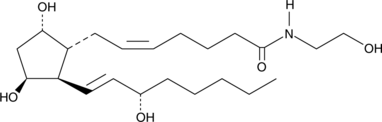
-
GC46412
11β-Prostaglandin F2α-d4
9α,11βPGF2αd4, 11βPGF2αd4, 11epi PGF2αd4
An internal standard for the quantification of 11β-PGF F2α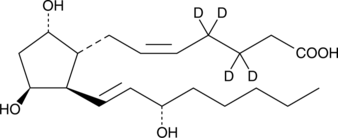
-
GC40422
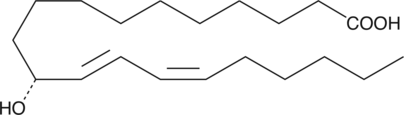
11(R)-HEDE
11(R)-HEDE is produced from 11Z,14Z-eicosadienoic acid by COX in a lipoxygenase-type reaction.
-
GC18660
11-dehydro Thromboxane B2
11-dehydro TXB2, 11-keto TXB2
Thromboxane B2 (TXB2) is released in substantial quantities from aggregating platelets and metabolized during circulation to 11-dehydro TXB2 and 2,3-dinor TXB2.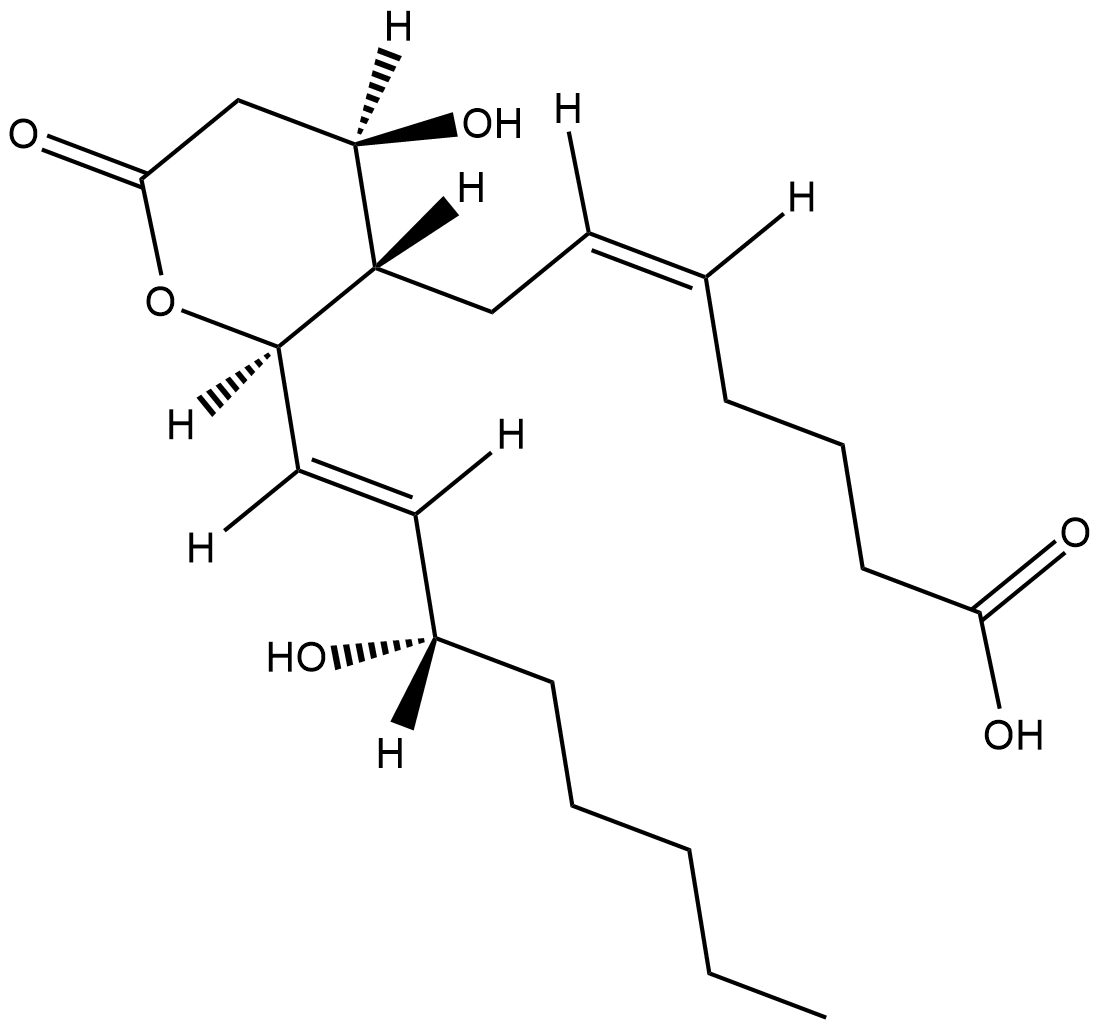
-
GC46408
11-dehydro Thromboxane B2-d4
11-dehydro TXB2-d4, 11keto TXB2d4
An internal standard for the quantification of 11dehydro thromboxane B2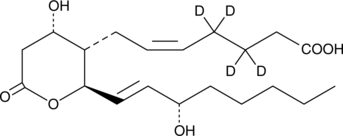
-
GC41877
11-dehydro Thromboxane B3
11dehydro TXB3
11-dehydro TXB3 is a urinary metabolite of TXA3 in humans with enhanced dietary intake of EPA.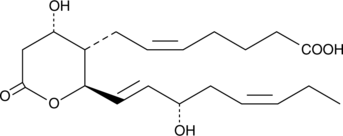
-
GC18634
11-deoxy Prostaglandin E1
11deoxy PGE1
11-deoxy Prostaglandin E1 (11-deoxy PGE1) is a synthetic analog of PGE1.
-
GC41121
11-deoxy Prostaglandin E2
11deoxy PGE2
11-deoxy Prostaglandin E2 (11-deoxy PGE2) is a stable, synthetic analog of PGE2.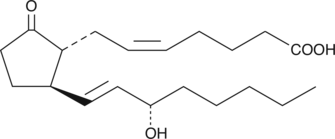
-
GC41401
11-deoxy Prostaglandin F1α
11deoxy PGF1α
11-deoxy PGF1α is a synthetic analog of PGF1α.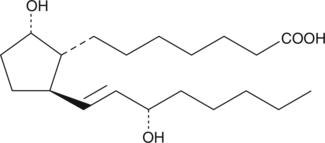
-
GC41402
11-deoxy Prostaglandin F1β
9β,11deoxy PGF1α, 11deoxy PGF1β
11-deoxy PGF1β is a synthetic analog of PGF1β.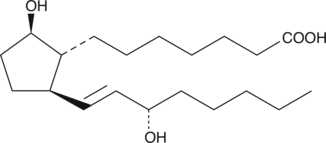
-
GC40274
11-deoxy Prostaglandin F2α
11deoxy PGF2α
11-deoxy PGF2α is a synthetic analog of PGF2α.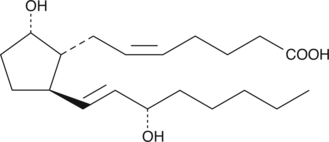
-
GC40275
11-deoxy Prostaglandin F2β
11deoxy PGF2β
11-deoxy Prostaglandin F2β (11-deoxy PGF2β) is an analog of PGF2β.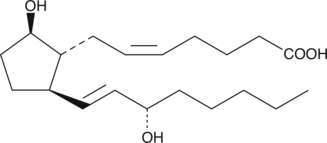
-
GC40335
11-deoxy-11-methylene Prostaglandin D2
11deoxy11methylene PGD2
Prostaglandin D2 is one of the five primary enzymatic prostaglandins derived directly from PGH2.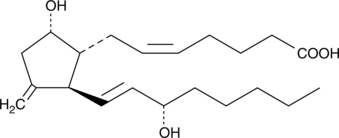
-
GC41879
11-deoxy-11-methylene-15-keto Prostaglandin D2
11deoxy11methylene15keto PGD2
Prostaglandin D2 (PGD2) is one of the five primary enzymatic prostaglandins derived directly from PGH2.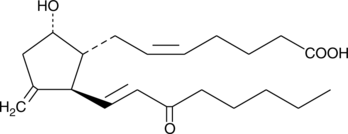
-
GC40390
11-deoxy-16,16-dimethyl Prostaglandin E2
11deoxy16,16dimethyl PGE2
11-deoxy-16,16-dimethyl PGE2 is a stable synthetic analog of PGE2.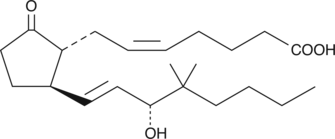
-
GC41880
11-keto Fluprostenol
Fluprostenol Prostaglandin D2
11-keto Fluprostenol is an analog of prostaglandin D2 (PGD2) with structural modifications intended to give it a prolonged half-life and greater potency.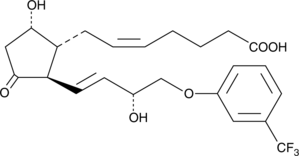
-
GC18617
11β-Prostaglandin E1
11βPGE1, 11epi PGE1
11β-Prostaglandin E1 (11β-PGE1) is an epimerized form of PGE1 at the C-11 position.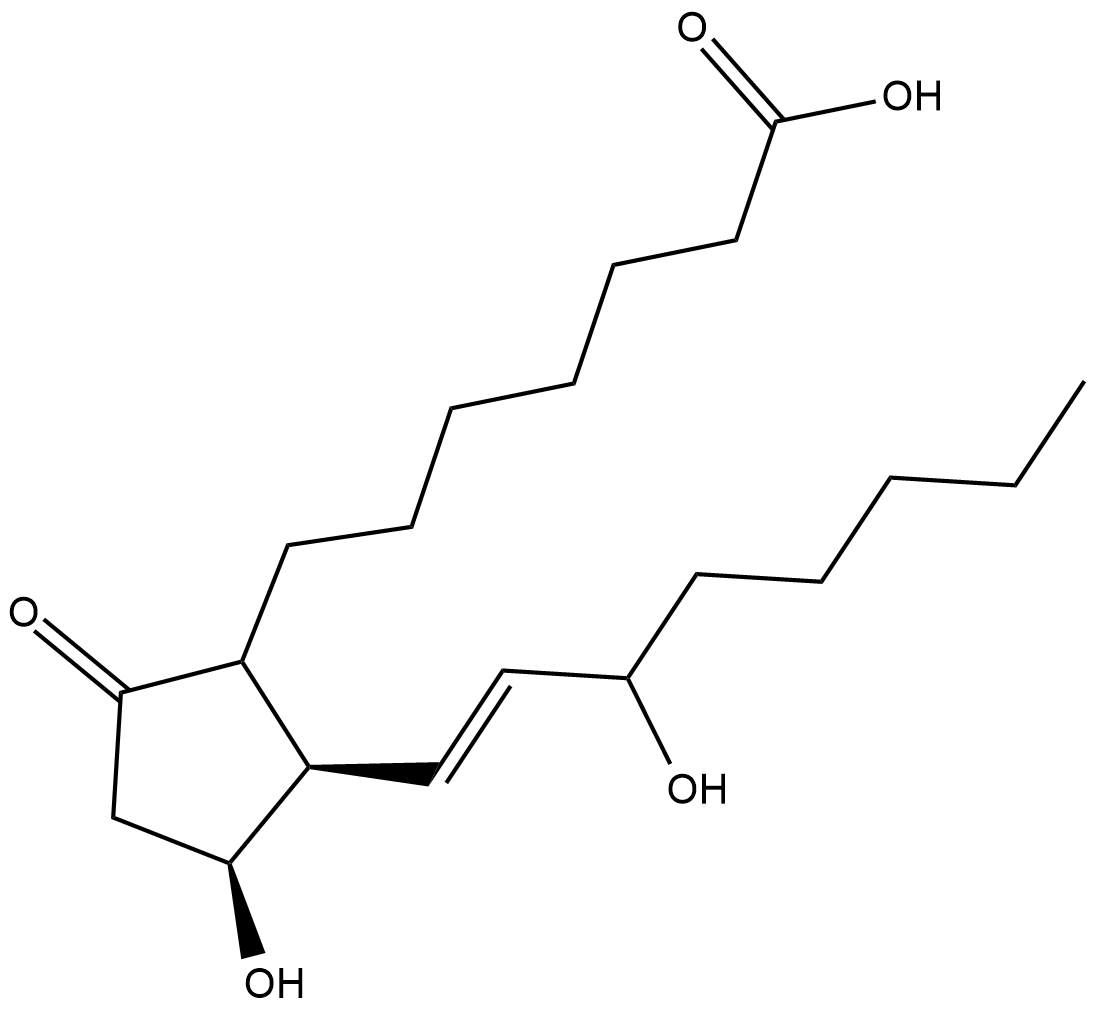
-
GC18637
11β-Prostaglandin F2α
9α,11βPGF2α, 11βPGF2α, 11epi PGF2α
11β-Prostaglandin F2α (11β-PGF2α) is the primary plasma metabolite of PGD2 in vivo.
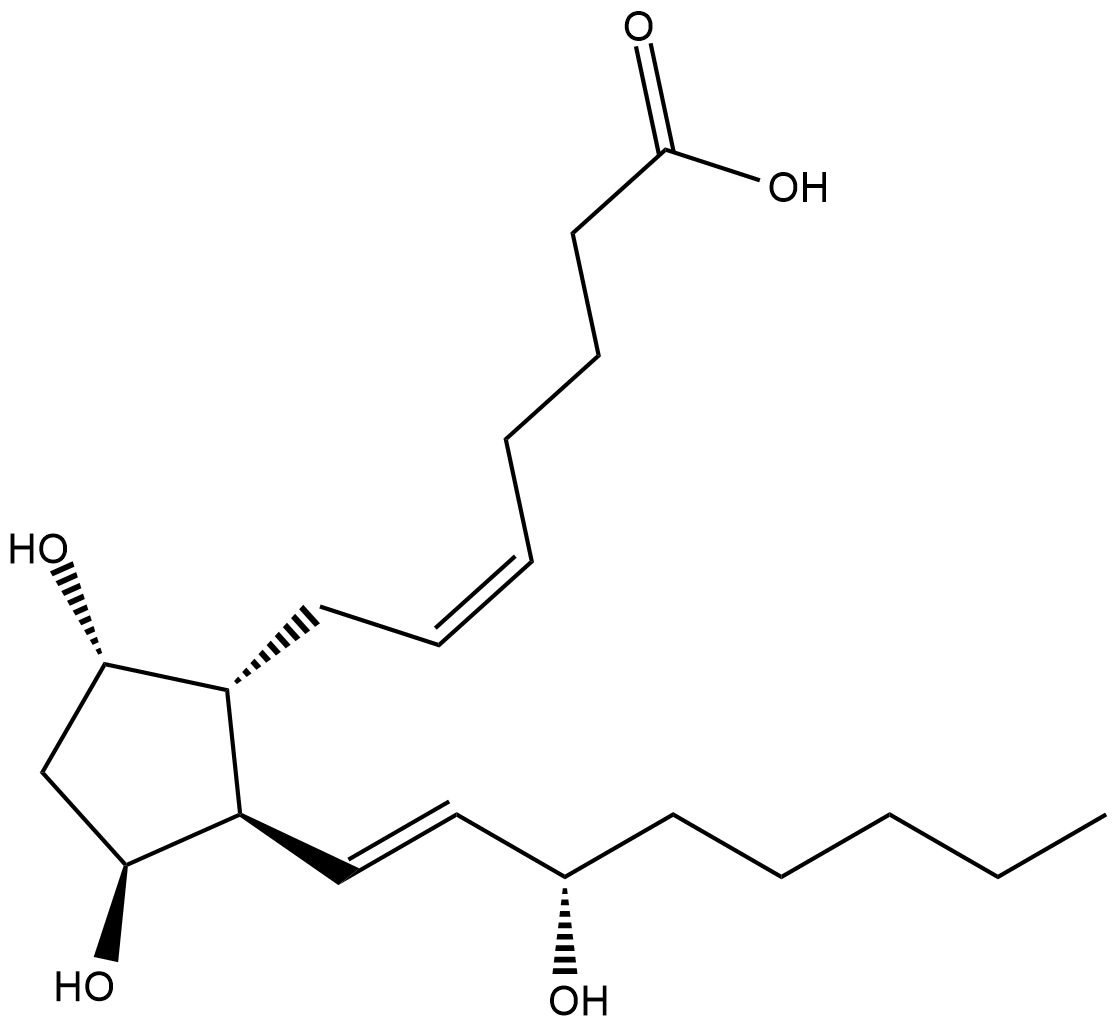
-
GC19462
13(R)-HODE
13(R)-HODE is the opposite enantiomer of the 13(S)-HODE produced when linoleic acid is incubated with soybean lipoxygenase.

-
GC46420
13(S)-HODE-d4
An internal standard for the quantification of 13-HODE
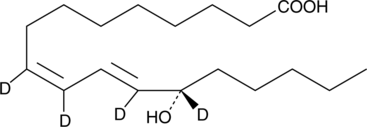
-
GC40745
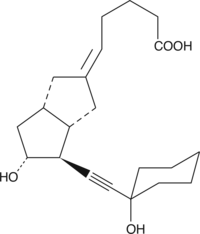
13,14-dehydro-15-cyclohexyl Carbaprostacyclin
13,14-dehydro-15-cyclohexyl Carbaprostacyclin is a chemically stable analog of PGI2.
-
GC41433
13,14-dihydro Prostaglandin E1
PGE0, 13,14-dihydro PGE1
13,14-dihydro Prostaglandin E1 (13,14-dihydro PGE1) is a biologically active metabolite of PGE1 with comparable potency to the parent compound.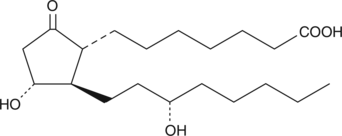
-
GC41902
13,14-dihydro Prostaglandin F1α
PGFα, 13,14dihydro PGF1α
13,14-dihydro PGF1α is a potential metabolite of PGF1α.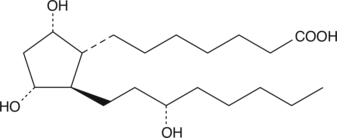
-
GC41434
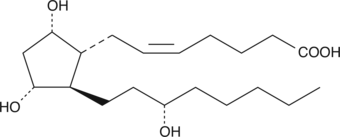
13,14-dihydro Prostaglandin F2α
13,14-dihydro PGF2α
13,14-dihydro Prostaglandin F2α (13,14-dihydro PGF2α) is the analog of PGF2α which has no unsaturation in the lower side chain.
-
GC41435
13,14-dihydro-15(R)-Prostaglandin E1
13,14dihydro15(R)PGE1
13,14-dihydro-15(R)-Prostaglandin E1 (13,14-dihydro-15(R)-PGE1) is an analog of 13,14-dihydro-PGE1 , which has the hydroxyl group at C-15 in the unnatural R configuration.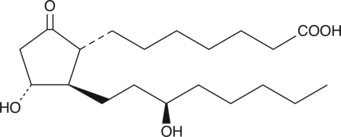
-
GC41097
13,14-dihydro-15-keto Prostaglandin A2
13,14-dihydro-15-keto PGA2
PGE2 is metabolized rapidly to 13,14-dihydro-15-keto PGE2, which is present in the plasma of humans and other mammals.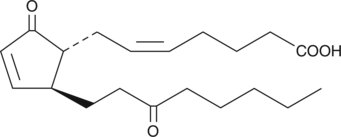
-
GC40578
13,14-dihydro-15-keto Prostaglandin D1
13,14dihydro15keto PGD1
Prostaglandin D1 (PGD1) is the theoretical D-series metabolite of dihomo-γ-linolenic acid (DGLA), but to date it has not been isolated as a natural product.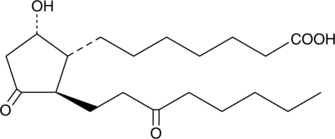
-
GC41411
13,14-dihydro-15-keto Prostaglandin D2
13,14-dihydro-15-keto PGD2
13,14-dihydro-15-keto Prostaglandin D2 (13,14-dihydro-15-keto PGD2) is a metabolite of PGD2 which is formed through the 15-hydroxy PGDH pathway.
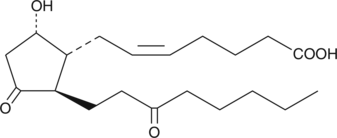
-
GC18783
13,14-dihydro-15-keto Prostaglandin E1
11α-Hydroxy-9,15-diketoprostanoic Acid, 15-keto-PGE0, 13,14-dihydro-15-keto PGE1, 15-keto-dihydro-PGE1, 15-keto Prostaglandin E0
13,14-dihydro-15-keto Prostaglandin E1 (PGE1) is a metabolite of PGE1 with much reduced biological activity.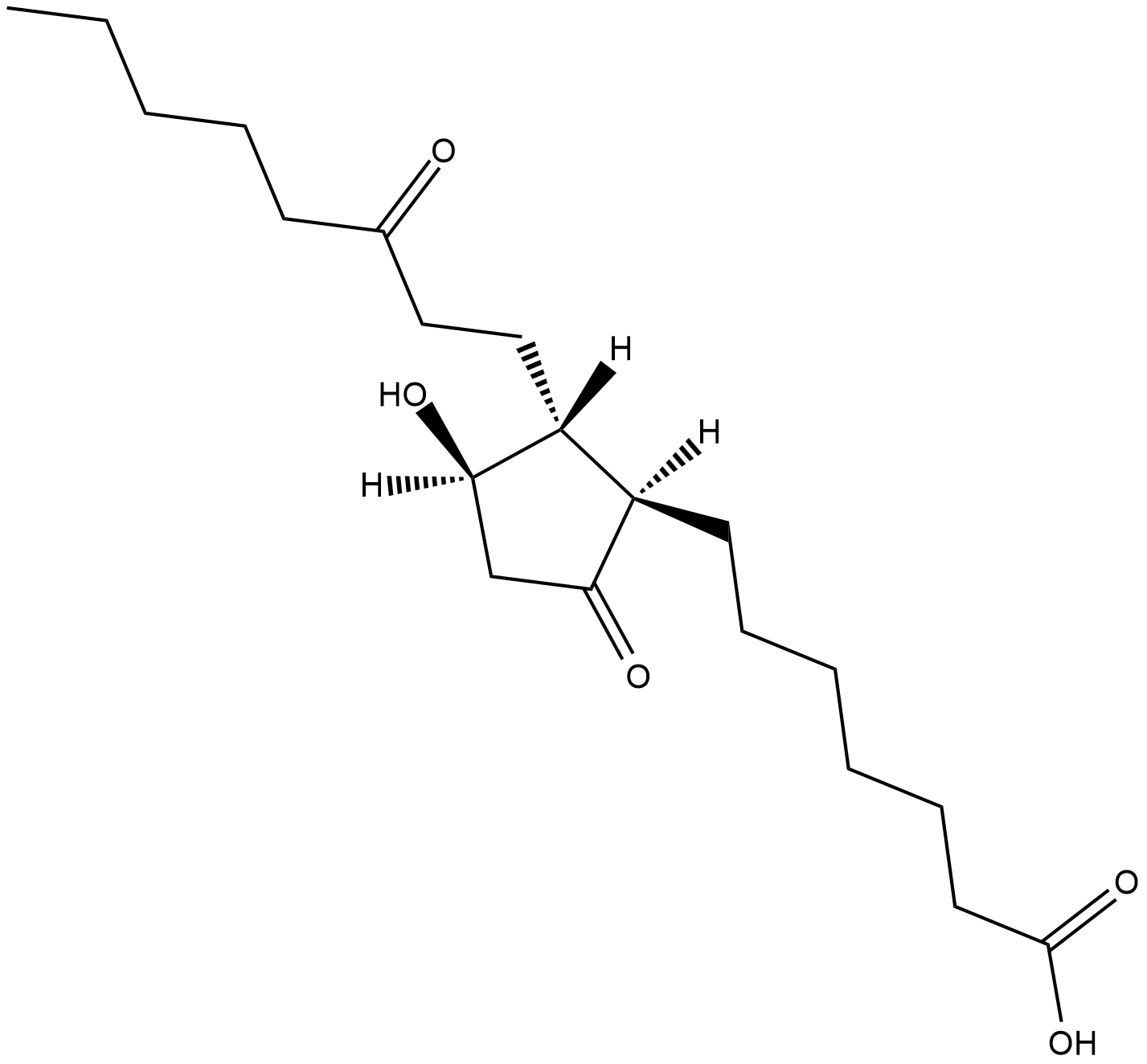
-
GC41413
13,14-dihydro-15-keto Prostaglandin E2
13,14-dihydro-15-keto PGE2
13,14-dihydro-15-keto Prostaglandin E2 (13,14-dihydro-15-keto PGE2) is the primary metabolite of PGE2 in plasma.
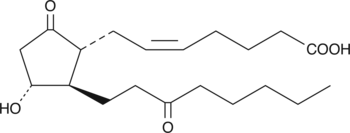
-
GC41436
13,14-dihydro-15-keto Prostaglandin F1α
13,14dihydro15keto PGF1α
13,14-dihydro-15-keto Prostaglandin F1α (13,14-dihydro-15-keto PGF1α) is a metabolite of PGF1α that has been reported in the rat stomach.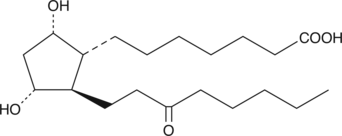
-
GC40579
13,14-dihydro-15-keto Prostaglandin F2α
13,14dihydro15keto PGF2α, PGFM
13,14-dihydro-15-keto Prostaglandin F2α (13,14-dihydro-15-keto PGF2α) is the first prominent plasma metabolite of PGF2α in the 15-hydroxy PGDH pathway.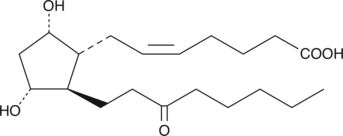
-
GC46429
13,14-dihydro-15-keto Prostaglandin F2α-d4
PGFMd4
An internal standard for the quantification of 13,14dihydro15keto PGF2α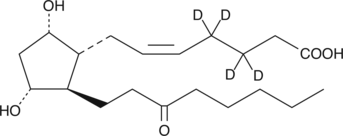
-
GC40625
13,14-dihydro-15-keto-tetranor Prostaglandin D2
A common metabolic pathway for several prostaglandins (PGs), including PGD2, involves the reduction of the double bond between C-13 and C-14 and oxidation of the hydroxyl group at C-15, producing 13,14-dihydro-15-keto PGs.
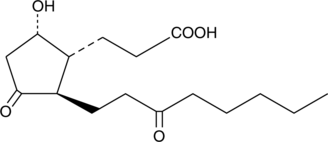
-
GC40626
13,14-dihydro-15-keto-tetranor Prostaglandin E2
13,14-dihydro-15-keto-tetranor PGE2
A common metabolic pathway for several prostaglandins (PG), including PGE2, involves the reduction of the double bond between C-13 and C-14 and oxidation of the hydroxyl group at C-15, producing 13,14-dihydro-15-keto PGs.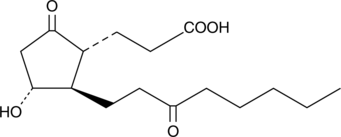
-
GC41903
13,14-dihydro-15-keto-tetranor Prostaglandin F1α
5β, 7αdihydroxy11ketotetranorProstanoic Acid, 9β13,14dihydro15ketotetranor Prostaglandin F1α, 9β,11αdihydroxy15oxo2,3,4,5tetranorProstanoic Acid
The metabolism of F series prostaglandins (PGs), including PGF1α and PGF2α, commonly begins with the reduction of the double bond between C-13 and C-14 and oxidation of the hydroxyl group at C-15, producing 13,14-dihydro-15-keto PGs.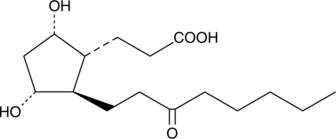
-
GC41904
13,14-dihydro-15-keto-tetranor Prostaglandin F1β
5β, 7αdihydroxy11ketotetranorProstanoic Acid, 9β13,14dihydro15ketotetranor Prostaglandin F1α, 9β,11αdihydroxy15oxo2,3,4,5tetranorProstanoic Acid
13,14-dihydro-15-keto tetranor-Prostaglandin F1β is a major urinary metabolite of PGE2 that is excreted in guinea pig urine at a concentration range of 1.34-2.74 μg/kg.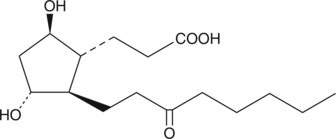
-
GC41905
13,14-dihydro-16,16-difluoro Prostaglandin E1
15hydroxy Lubiprostone
Prostaglandin E1 (PGE1) is produced by the metabolism of dihomo-γ-linolenic acid (DGLA) by the cyclooxygenase pathway.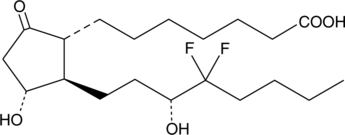
-
GC41472
15(R),19(R)-hydroxy Prostaglandin E2
15(R),19(R)hydroxy PGE2
19(R)-Hydroxylated prostaglandins occur in μg/ml concentrations in the semen of certain mammalian species, especially primates.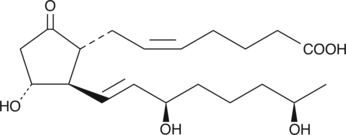
-
GC40532
15(R),19(R)-hydroxy Prostaglandin F2α
15(R),19(R)hydroxy PGF2α
19(R)-Hydroxylated prostaglandins (PGs) occur in μg/ml concentrations in the semen of certain mammalian species, especially primates.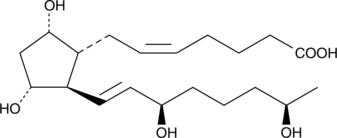
-
GC41921
15(R)-15-methyl Prostaglandin A2
15(R)15methyl PGA2
Arbaprostil (15(R)-15-methyl prostaglandin E2) is a synthetic prostaglandin E2 (PGE2) analog developed for its cytoprotective activity.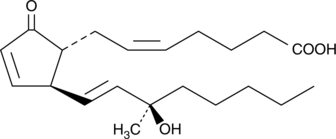
-
GC41164
15(R)-15-methyl Prostaglandin D2
15(R)15methyl PGD2
15(R)-15-methyl Prostaglandin D2 (15(R)-15-methyl PGD2) is a metabolically stable synthetic analog of PGD2.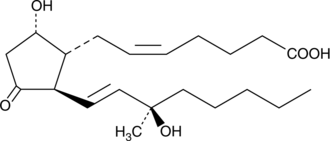
-
GC41165
15(R)-15-methyl Prostaglandin E2
Arbaprostil, 15(R)15methyl PGE2
15(R)-15-methyl Prostaglandin E2 (15(R)-15-methyl PGE2) is a prodrug for the potent PGE2 analog 15(S)-15-methyl PGE2.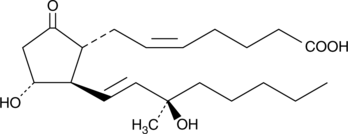
-
GC41440
15(R)-15-methyl Prostaglandin F2α
15(R)15methyl PGF2α
15(R)-15-methyl PGF2α is a metabolically stable analog of PGF2α.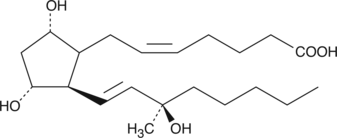
-
GC41244
15(R)-15-methyl Prostaglandin F2α methyl ester
15(R)-Methyl carboprost, 15(R)15methyl PGF2α methyl ester
15(R)-15-methyl Prostaglandin F2α methyl ester (15(R)-15-methyl PGF2α methyl ester) is a lipid soluble prodrug form of 15(R)-15-methyl PGF2α with increased membrane permeability.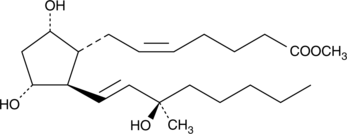
-
GC40988
15(R)-17-phenyl trinor Prostaglandin F2α
15epi Bimatoprost (free acid), 15(R)Bimatoprost (free acid), 15(R)17phenyl trinor PGF2α
17-phenyl trinor Prostaglandin F2α N-ethyl amide (17-phenyl trinor PGF2α) is an F-series prostaglandin analog which has been approved for use as an ocular hypotensive drug, sold under the Allergan trade name 17-phenyl trinor PGF2α ethyl amide.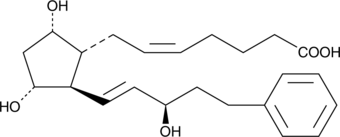
-
GC40648
15(R)-17-phenyl trinor Prostaglandin F2α ethyl amide
15(R)Bimatoprost, 15(R)17phenyl trinor PGF2α ethyl amide
15(R)-17-phenyl trinor Prostaglandin F2α ethyl amide (15(R)-17-phenyl trinor PGF2α ethyl amide) is an isomer of bimatoprost, characterized by an inverted (β) hydroxyl group at C-15.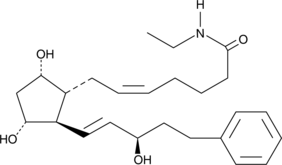
-
GC41922
15(R)-17-phenyl trinor Prostaglandin F2α isopropyl ester
15epi Bimatoprost isopropyl ester, 15(R)Bimatoprost isopropyl ester, 15(R)17phenyl trinor PGF2α isopropyl ester
15(R)-17-phenyl trinor Prostaglandin F2α isopropyl ester (15(R)-17-phenyl trinor PGF2α isopropyl ester) is the latanoprost-related isomer containing both a double bond at 13,14 and an inverted (β) hydroxyl group at C-15.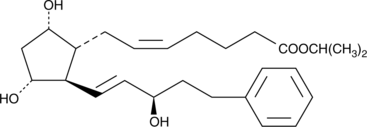
-
GC40972
15(R)-Iloprost
Iloprost is a second generation structural analog of prostacyclin (PGI2) with about ten-fold greater potency than the first generation stable analogs, typified by carbaprostacyclin.
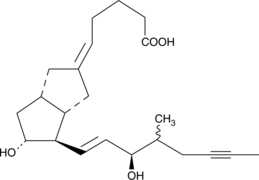
-
GC41173
15(R)-Pinane Thromboxane A2
15-epi-Pinane Thromboxane A2, 15-iso-Pinane Thromboxane A2, 15(R)PTA2, 15-epi-PTA2, 15-iso-PTA2
15(R)-Pinane thromboxane A2 is the (R)-epimer of pinane thromboxane A2.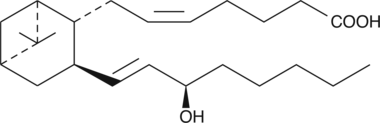
-
GC41416
15(R)-Prostaglandin D2
15(R)PGD2
Many of the effects of prostaglandin D2 (PGD2) are transduced via a traditional 7-transmembrane GPCR, the DP1 receptor.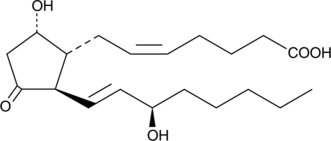
-
GC18735
15(R)-Prostaglandin E1
15(R)PGE1, 15epi PGE1
15(R)-PGE1 is the unnatural-C-15 stereoisomer of PGE1.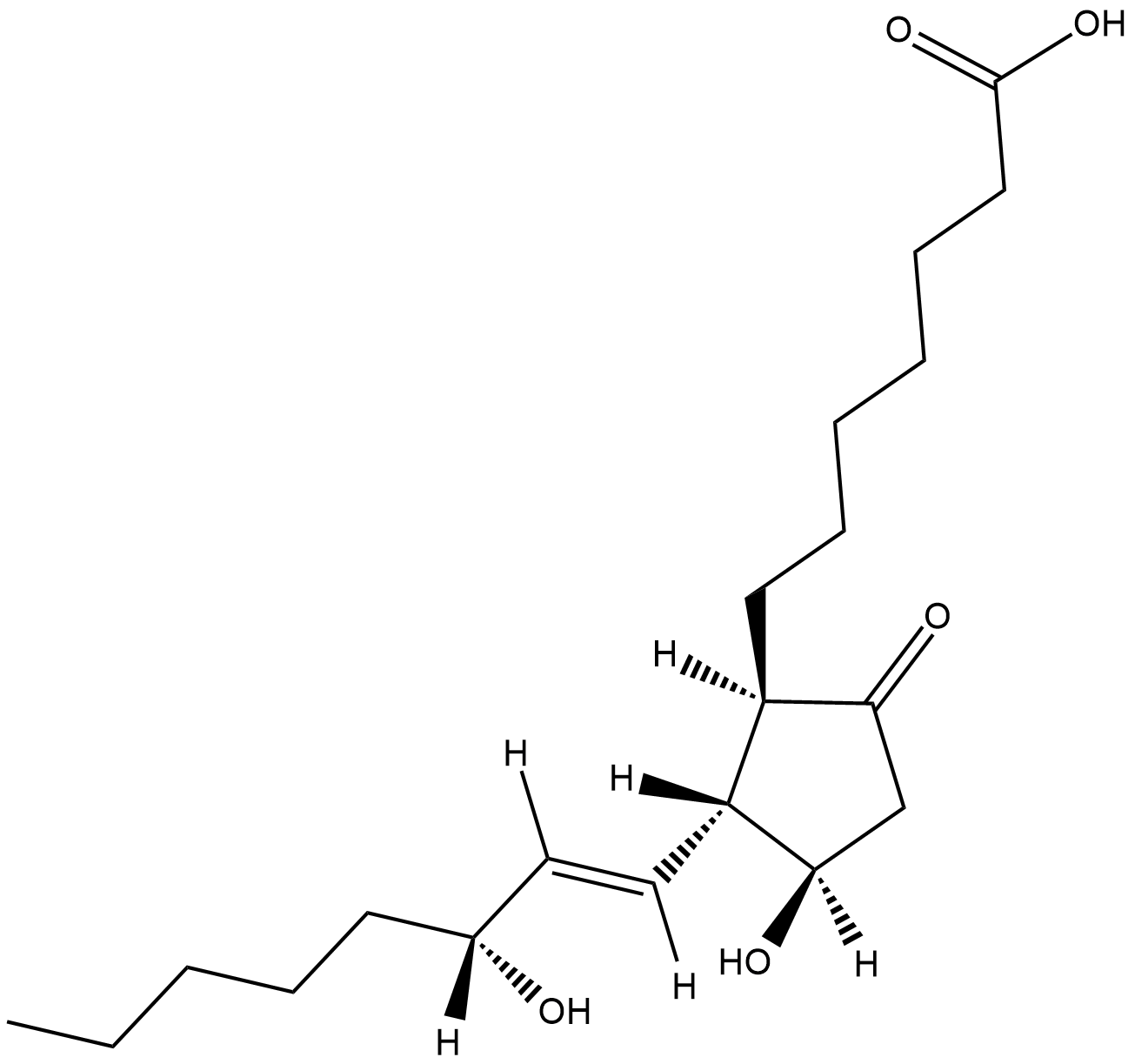
-
GC41417
15(R)-Prostaglandin E2
15(R)PGE2, 15epi PGE2
15(R)-Prostaglandin E2 (15(R)-PGE2) is the C-15 epimer of the naturally occurring 15(S)-PGE2.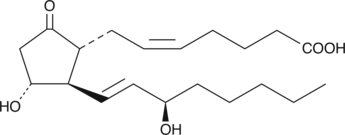
-
GC46439
15(R)-Prostaglandin F1α
15(R)-PGF1α, 15-epi PGF1α
The C-15 epimer of PGF1α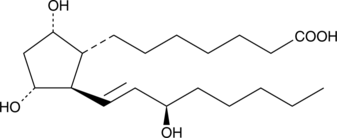
-
GC40581
15(R)-Prostaglandin F2α
15epi PGF2α
15(R)-PGF2α is the C-15 epimer of the naturally occurring mammalian autacoid PGF2α.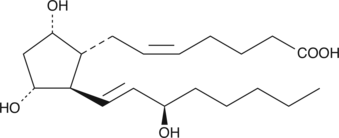
-
GC41166
15(S)-15-methyl Prostaglandin D2
15(S)15methyl PGD2
15(S)-15-methyl Prostaglandin D2 (15(S)-15-methyl PGD2) is a metabolically stable synthetic analog of PGD2.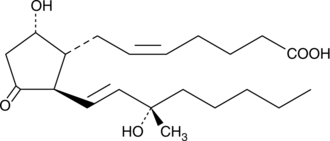
-
GC18767
15(S)-15-methyl Prostaglandin E1
15(S)15methyl PGE1
15(S)-15-methyl Prostaglandin E1 (15(S)-15-methyl PGE1) is a metabolically stable synthetic analog of PGE1 .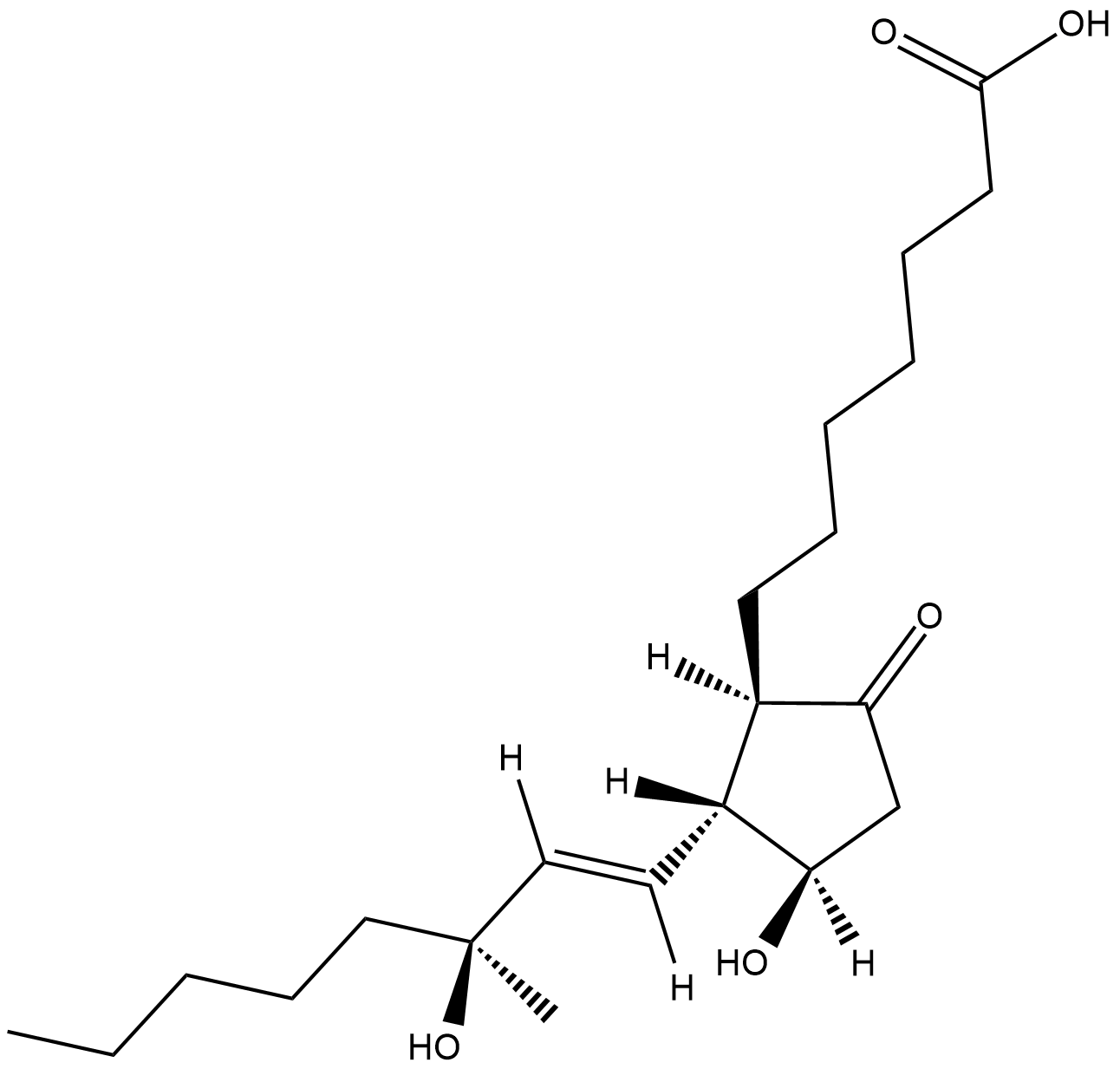
-
GC41167
15(S)-15-methyl Prostaglandin E2
15(S)15methyl PGE2
15(S)-15-methyl PGE2 is a potent, metabolically stable analog of PGE2.
-
GC41924
15(S)-15-methyl Prostaglandin F2α isopropyl ester
15(S)15methyl PGF2α isopropyl ester
15(S)-15-methyl Prostaglandin F2α (15(S)-15-methyl PGF2α) has been shown to have potent uterine stimulant and abortifacient properties when administered intramuscularly to induce labor.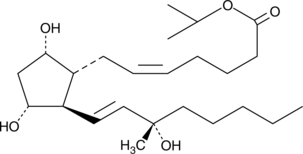
-
GC18372
15(S)-15-methyl Prostaglandin F2α methyl ester
Methyl carboprost, 15(S)15methyl PGF2α methyl ester, U36384
15(S)-15-methyl PGF2α methyl ester is a derivative of 15(S)-15-methyl PGF2α with increased membrane permeability.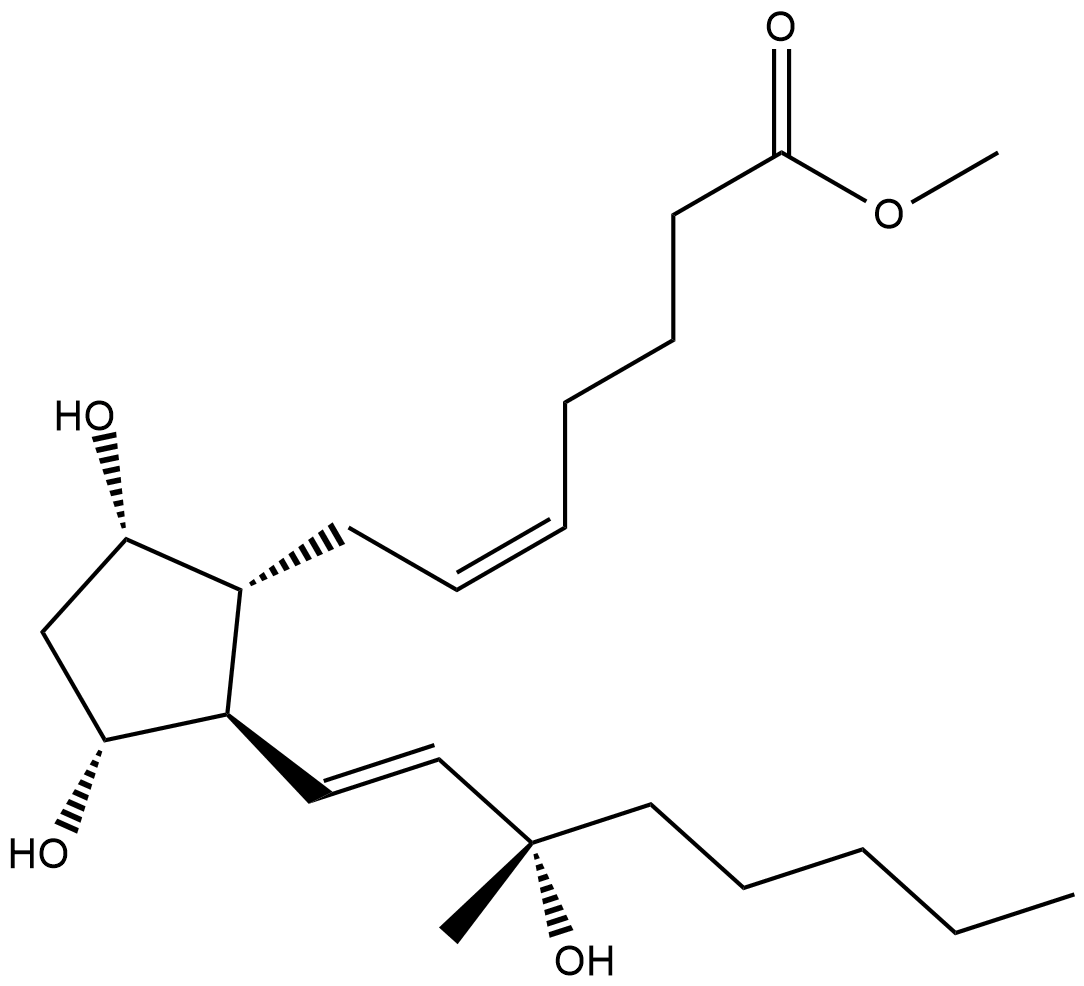
-
GC40283
15(S)-Fluprostenol
(+)-15(R)-Fluprostenol isopropyl ester is a prodrug (Travoprost) which is converted by esterase enzymatic activity in the cornea to yield the corresponding free acid.
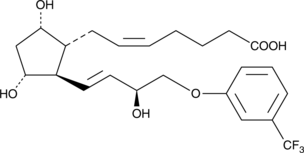
-
GC41589
15(S)-Fluprostenol isopropyl ester
15(S)FluIpr
15(S)-Fluprostenol isopropyl ester (15(S)-Flu-Ipr) is the unnatural C-15 epimer of Travoprost.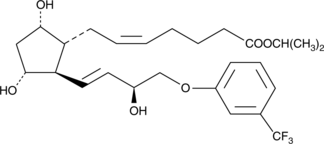
-
GC46442
15(S)-HETE-d8
15(S)-Hydroxyeicosatetraenoic Acid-d8
An internal standard for the quantification of 15-HETE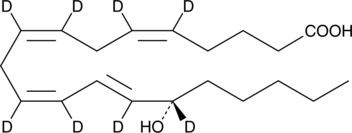
-
GC41093
15(S)-Latanoprost
15epi Latanoprost
15(S)-Latanoprost is an analog of latanoprost in which the hydroxyl at carbon 15 is inverted relative to latanoprost.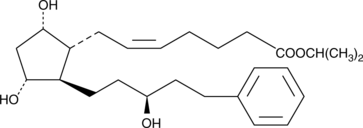
-
GC49036
15-(6-nitroxyhexanoyl)-17-phenyl trinor Prostaglandin F2α
15-(6-nitroxyhexanoyl)-17-phenyl trinor PGF2α, 15-(6-nitroxyhexanoyl)-Bimatoprost, NCX 470
A nitric oxide-donating derivative of 17-phenyl trinor prostaglandin F2α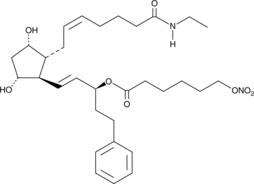
-
GC41168
15-cyclohexyl pentanor Prostaglandin F2α
15cyclohexyl pentanor PGF2α
15-cyclohexyl pentanor PGF2α is an analog of PGF2α with resistance to 15-hydroxy PGDH metabolism.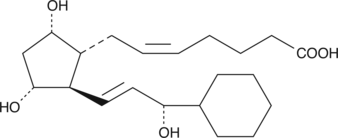
-
GC40375
15-deoxy-δ12,14-Prostaglandin A1
15deoxyΔ12,14PGA1
15-deoxy-δ12,14-Prostaglandin A1 (15-deoxy-δ12,14-PGA1) is a synthetic PGA1 analog.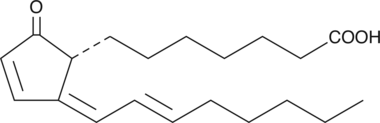
-
GC40350
15-deoxy-δ12,14-Prostaglandin A2
15deoxyΔ12,14PGA2
15-deoxy-δ12,14-PGA2 is a synthetic analog of PGA2.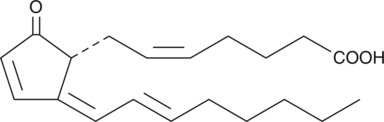
-
GC41928
15-deoxy-δ12,14-Prostaglandin J2 Glutathione
15deoxyΔ12,14PGJ2 Glutathione
15-deoxy-δ12,14-Prostaglandin J2 Glutathione (15-deoxy-δ12,14-PGJ2 Glutathione) is a non-enzymatic adduct formed from 15-deoxy-δ12,14-PGJ2 and glutathione.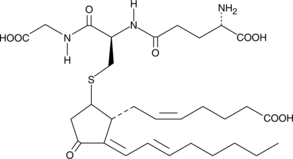
-
GC46447
15-deoxy-δ12,14-Prostaglandin J2-d4
15deoxyΔ12,14PGJ2d4
An internal standard for the quantification of 15deoxyΔ12,14prostaglandin J2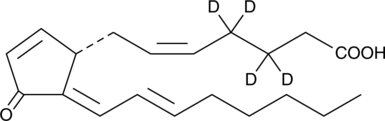
-
GC41125
15-epi Prostaglandin A1
15epi PGA1
15-epi PGA1 is the 15(R) stereoisomer of PGA1.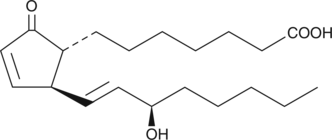
-
GC41933
15-keto Fluprostenol isopropyl ester
15keto Travoprost
15-keto Fluprostenol isopropyl ester (15-Keto Fluprostenol isopropyl ester) هو مستقلب من Travoprost.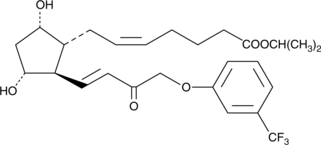
-
GC41934
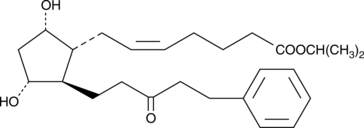
15-keto Latanoprost
15-Keto latanoprost هو مستقلب من Latanoprost ، وهو عامل خافض لضغط العين
-
GC40989
15-keto Latanoprost (free acid)
15-keto Latanoprost is a potential metabolite of latanoprost when administered to animals.
-
GC41103
15-keto Prostaglandin A1
15keto PGA1
Prostaglandin A1 (PGA1) was first isolated as a dehydration product of the PGE1 compounds found in human semen.