MRIA9 |
| رقم الكتالوجGC62657 |
MRIA9 عبارة عن مثبط كيناز (SIK) ومثبط PAK2 / 3 تنافسي لـ ATP ، مع قيم IC50 لـ 516 نانومتر و 180 نانومتر و 127 نانومتر لـ SIK1 و SIK2 و SIK3 ، على التوالي
Products are for research use only. Not for human use. We do not sell to patients.
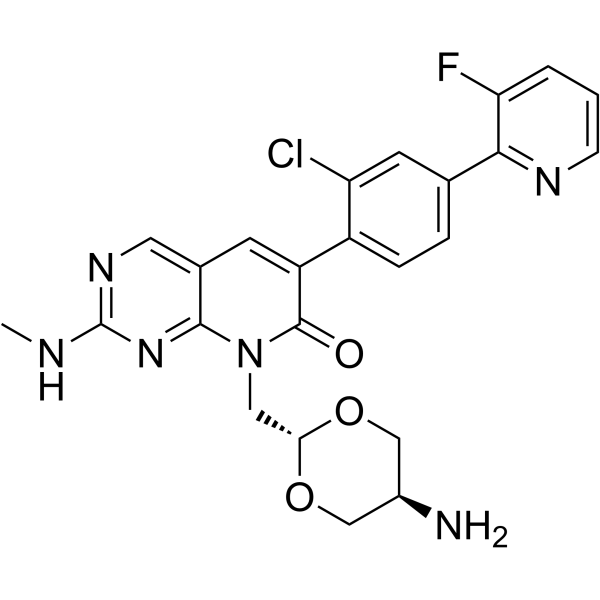
Cas No.: 2750707-05-0
Sample solution is provided at 25 µL, 10mM.
MRIA9 is an ATP-competitive, pan Salt-Inducible kinase (SIK) and PAK2/3 inhibitor, with IC50 values of 516 nM, 180 nM and 127 nM for SIK1, SIK2 and SIK3, respectively[1].
MRIA9 (5 μM) MRIA9 sensitizes SKOV3 cells to paclitaxel treatment through inducing pronounced apoptosis[1].MRIA9 (5 μM) with paclitaxel (2 nM) significantly enhances cell death in HeLa cells[1].MRIA9 strongly impedes centrosome function, causes mitotic spindle mispositioning in ovarian cancer cell lines, prevents the centrosome disjunction during the late G2 phase, and sensitized ovarian cancer cells and patient derived 3D-spheroids to paclitaxel treatment[2].
MRIA9 shows high oral bioavailability (F = 75-80%)[1].
[1]. Roberta Tesch, et al. Structure-Based Design of Selective Salt-Inducible Kinase Inhibitors. J Med Chem. 2021 Jun 24;64(12):8142-8160.
[2]. Monika Raab, et al. The Small-Molecule Inhibitor MRIA9 Reveals Novel Insights into the Cell Cycle Roles of SIK2 in Ovarian Cancer Cells. Cancers 2021, 13(15), 3658.
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















