Ombrabulin hydrochloride (AVE8062) (Synonyms: AC-7700, AVE-8062, CS 39-L-Ser) |
| رقم الكتالوجGC34022 |
Ombrabulin hydrochloride (AVE8062) هو مشتق من CA-4 فوسفات ، المعروف عنه آثاره المضادة للأوعية من خلال الاضطراب الانتقائي للهيكل الخلوي لتوبيولين للخلايا البطانية.
Products are for research use only. Not for human use. We do not sell to patients.
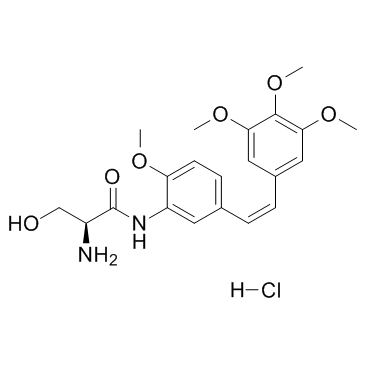
Cas No.: 253426-24-3
Sample solution is provided at 25 µL, 10mM.
Ombrabulin hydrochloride is a derivative of CA-4 phosphate, which is known to exhibit antivascular effects through selective disruption of the tubulin cytoskeleton of endothelial cells.
The effect of Ombrabulin (AVE8062) on endothelial or tumor cell viability is examined using the MTT assay. The IC50 of Ombrabulin for the mouse mesenteric endothelial cells (MMEC) is 10 nM and ranges between 7 and 20 nM for the tumor cell lines (HeyA8, SKOV3ip1, and HeyA8-MDR). Comparative analysis of the nonlinear least-squares regression of the dose-response curves for each agent alone and combination Ombrabulin /Docetaxel show a significantly lower IC50 than either agent alone (P<0.005, all cell lines). The cytotoxicity of Docetaxel is 2- to 4-fold greater in combination with Ombrabulin for the endothelial and tumor cells compared with Docetaxel alone[1].
Before performing therapy experiments, the tolerability of various doses of Ombrabulin (AVE8062) ranging from 10 to 100 mg/kg is tested given twice weekly via i.v., i.p., or s.c. routes in nude mice (n=3 per group). The i.v. and s.c. routes are not pursued further due to problems with skin or tail vein necrosis. The i.p. route is well tolerated with doses up to 100 mg/kg. Next, preliminary experiments are done to determine the lowest dose for in vivo therapeutic efficacy. Starting 7 days after tumor cell injection, nude mice (n=5 per group) bearing HeyA8 ovarian cancer cells are treated with either vehicle or Ombrabulin 10, 30, 50, and 100 mg/kg twice weekly i.p. for 3 weeks. There is 65% reduction in tumor weight in the 30 mg/kg group compared with the vehicle control group (P30 mg/kg are not significantly better; therefore, the 30 mg/kg dose is selected for subsequent therapy experiments[1].
[1]. Kim TJ, et al. Antitumor and antivascular effects of AVE8062 in ovarian carcinoma. Cancer Res. 2007 Oct 1;67(19):9337-45.
Average Rating: 5 (Based on Reviews and 27 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















