4-iodo-SAHA (Synonyms: ISAHA) |
| رقم الكتالوجGC17005 |
4-Iodo-SAHA (1k) عبارة عن مثبط نشط عن طريق الفم من الفئة الأولى والفئة الثانية من مثبطات هيستون ديستيلاز (HDAC) مع EC50s 1.1 و 0.95 و 0.12 و 0.24 و 0.85 و 1.3 ميكرومتر لخطوط الخلايا Skbr3 و HT29 و U937 و JA16 و HL60 ، على التوالى. يمكن استخدام 4-Iodo-SAHA (1 ك) لأبحاث السرطان
Products are for research use only. Not for human use. We do not sell to patients.
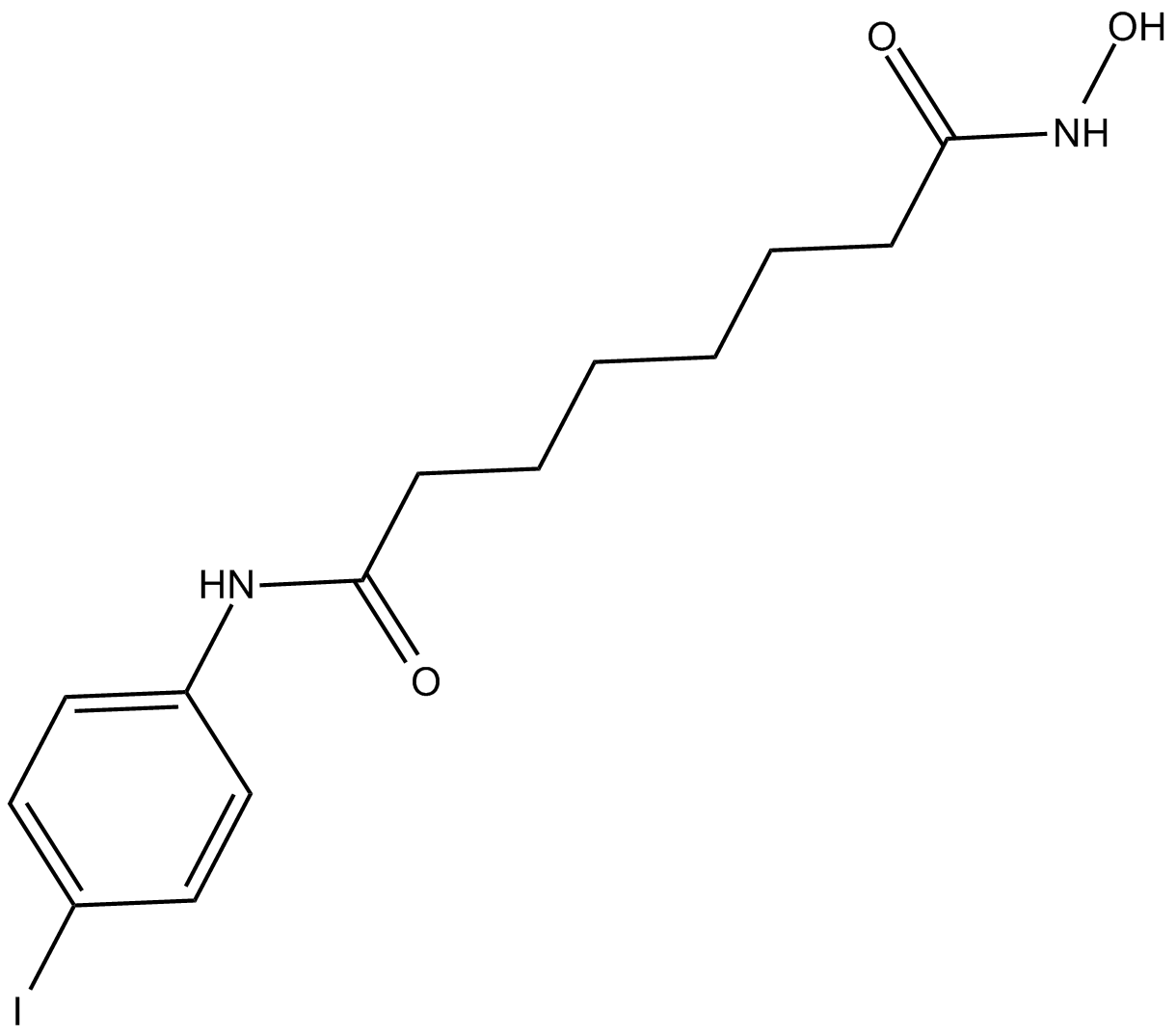
Cas No.: 1219807-87-0
Sample solution is provided at 25 µL, 10mM.
4-iodo-SAHA is a hydrophobic derivative of SAHA, the class I and class II histone deacetylase (HDAC) inhibitor [1].
The reversible acetylation of lysine residues in histone plays an important role in transcriptional activation and repression. The regulation of these post-translational modifications is balanced by histone acetyltransferase (HAT) and histone deacetylase (HDAC) activities. HDACs are also involved in reversible acetylation of non-histone proteins [1].
4-iodo-SAHA is a histone deacetylase (HDAC) inhibitor. In SKBR3-breast-derived cell line, 4-iodo-SAHA inhibited cell proliferation with EC50 value of 1.1 μM. In HT29 colon-derived cell line, leukemia-derived U937 tumor cell line, JA16, HL60 and K562 cell lines, 4-iodo-SAHA inhibited cell proliferation with EC50 values of 0.95, 0.12, 0.24, 0.85 and 1.3 μM, respectively. 4-iodo-SAHA is 10-fold more potent as an inhibitor of U937 leukemia cell proliferation compared to SAHA (0.12 μM versus 1.2 μM). In SKBR3 cells, 4-iodo-SAHA reduced acetylated H4 and p21 levels [1].
Reference:
[1]. Salmi-Smail C, Fabre A, Dequiedt F, et al. Modified cap group suberoylanilide hydroxamic acid histone deacetylase inhibitor derivatives reveal improved selective antileukemic activity. J Med Chem. 2010 Apr 22;53(8):3038-47.
Average Rating: 5 (Based on Reviews and 11 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















