Rimantadine |
| رقم الكتالوجGC17819 |
ريمانتادين (فلومادين) دواء مضاد لفيروس الإنفلونزا
Products are for research use only. Not for human use. We do not sell to patients.
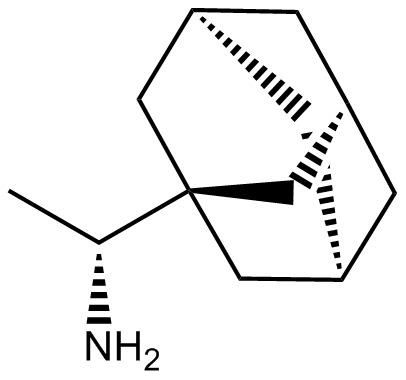
Cas No.: 13392-28-4
Sample solution is provided at 25 µL, 10mM.
Rimantadine (Flumadine) is an anti-influenza virus drug.Target: Influenza Virusrimantadine are oral antiviral drugs useful in the prophylaxis and treatment of influenza A virus infections. rimantadine has prophylactic efficacy comparable to amantadine but lower potential for causing adverse effects [1]. double-blind study of children with influenza-like illness. 37 received rimantadine for five days. Of the total 37 children in the rimantadine group, 27% were found to have resistant isolated compared with 6% in the total group receiving acetaminophen (P < .04). Furthermore, the mean inhibitory concentration of rimantadine increased with time in the rimantadine group (r = .4, P = .002) [2].
References:
[1]. Tominack, R.L. and F.G. Hayden, Rimantadine hydrochloride and amantadine hydrochloride use in influenza A virus infections. Infect Dis Clin North Am, 1987. 1(2): p. 459-78.
[2]. Hall, C.B., et al., Children with influenza A infection: treatment with rimantadine. Pediatrics, 1987. 80(2): p. 275-82.
Average Rating: 5 (Based on Reviews and 23 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















