Aleglitazar |
| Catalog No.GC35281 |
Aleglitazar (R1439) es un potente agonista dual de PPARα/γ, con IC50 de 38 nM y 19 nM para PPARa y PPARγ humanos, respectivamente.
Products are for research use only. Not for human use. We do not sell to patients.
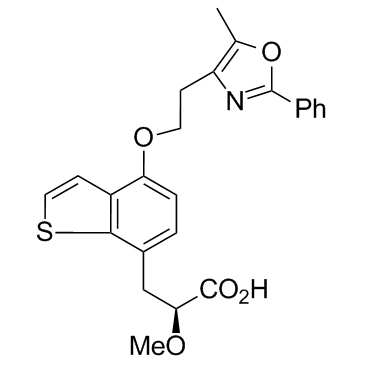
Cas No.: 475479-34-6
Sample solution is provided at 25 µL, 10mM.
Aleglitazar(R1439; RO-0728804) is a new dual PPAR-α/γ agonist with IC50 of 2.8 nM/4.6 nM.IC50 Value: 2.8 nM(PPAR-α); 4.6 nM(PPAR-γ)Target: PPARα/γAleglitazar (R1439) is a dual peroxisome proliferator-activated receptor (PPAR) agonist, with affinity to PPARα and PPARγ. Aleglitazar is being developed for the treatment of type II diabetes; It is currently in phase III clinical trials. In preliminary clinical studies, Aleglitazar has been demonstrated to improve hyperglycemia and dyslipidemia in patients with type 2 diabetes mellitus. Aleglitazar has beneficial effects on both lipid and glucose parameters and may have a therapeutic role in modifying cardiovascular risk factors and improving glycemic control in patients with T2DM. Aleglitazar combines the lipid benefits of fibrates and the insulin-sensitizing benefits of thiazolidinediones. PPARγ|19 nM (IC50)|PPARα|38 nM (IC50)
[1]. BÉnardeau A, Verry P, Atzpodien EA, et al. Effects of the dual PPAR-α/γ agonist aleglitazar on glycaemic control and organ protection in the Zucker diabetic fatty rat. Diabetes Obes Metab. 2013 Feb;15(2):164-74. [2]. Younk LM, Uhl L, Davis SN. Pharmacokinetics, efficacy and safety of aleglitazar for the treatment of type 2 diabetes with high cardiovascular risk. Expert Opin Drug Metab Toxicol. 2011 Jun;7(6):753-63. [3]. Foley-Comer AJ, Young AM, Russell-Yarde F, et al. Aleglitazar, a balanced PPARα/γ agonist, has no clinically relevant pharmacokinetic interaction with high-dose atorvastatin or rosuvastatin. Expert Opin Investig Drugs. 2011 Jan;20(1):3-12. [4]. Cavender MA, Lincoff AM. Therapeutic potential of aleglitazar, a new dual PPAR-α/γ agonist: implications for cardiovascular disease in patients with diabetes mellitus. Am J Cardiovasc Drugs. 2010;10(4):209-16. [5]. BÉnardeau A, Benz J, Binggeli A, et al. Aleglitazar, a new, potent, and balanced dual PPARalpha/gamma agonist for the treatment of type II diabetes. Bioorg Med Chem Lett. 2009 May 1;19(9):2468-73.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















