Amifampridine |
| Catalog No.GC35318 |
La amifampridina (3,4-diaminopiridina) es un bloqueador de los canales de potasio (Kv) controlado por voltaje (PCB) activo por vÍa oral, potente y permeable a las células.
Products are for research use only. Not for human use. We do not sell to patients.
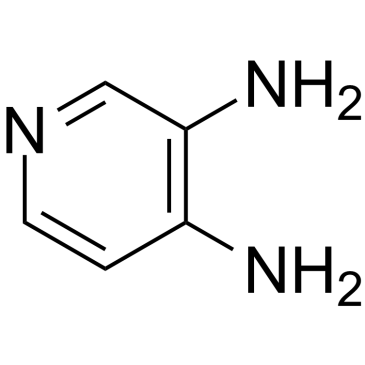
Cas No.: 54-96-6
Sample solution is provided at 25 µL, 10mM.
Amifampridine (3,4-Diaminopyridine) is a drug, predominantly in the treatment of a number of rare muscle diseases.Target: OthersAmifampridine is a drug, predominantly in the treatment of a number of rare muscle diseases. Amifampridine works by blocking potassium channel efflux in nerve terminals so that action potential duration is increased. Ca2+ channels can then be open for a longer time and allow greater acetylcholine release to stimulate muscle at the end plate. A 2005 systematic review from the Cochrane Collaboration found some data favouring its use in LEMS. Amifampridine is also used to treat many of the congenital myasthenic syndromes, particularly those with defects in choline acetyltransferase, downstream kinase 7, and those where any kind of defect causes "fast channel" behaviour of the acetylcholine receptor. From Wikipedia.
[1]. http://en.wikipedia.org/wiki/3,4-Diaminopyridine#cite_note-4
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















