Risperidone (Synonyms: Apexidone, Psychodal, R 64766) |
| Catalog No.GC12986 |
Inhibidor de SR-2A
Products are for research use only. Not for human use. We do not sell to patients.
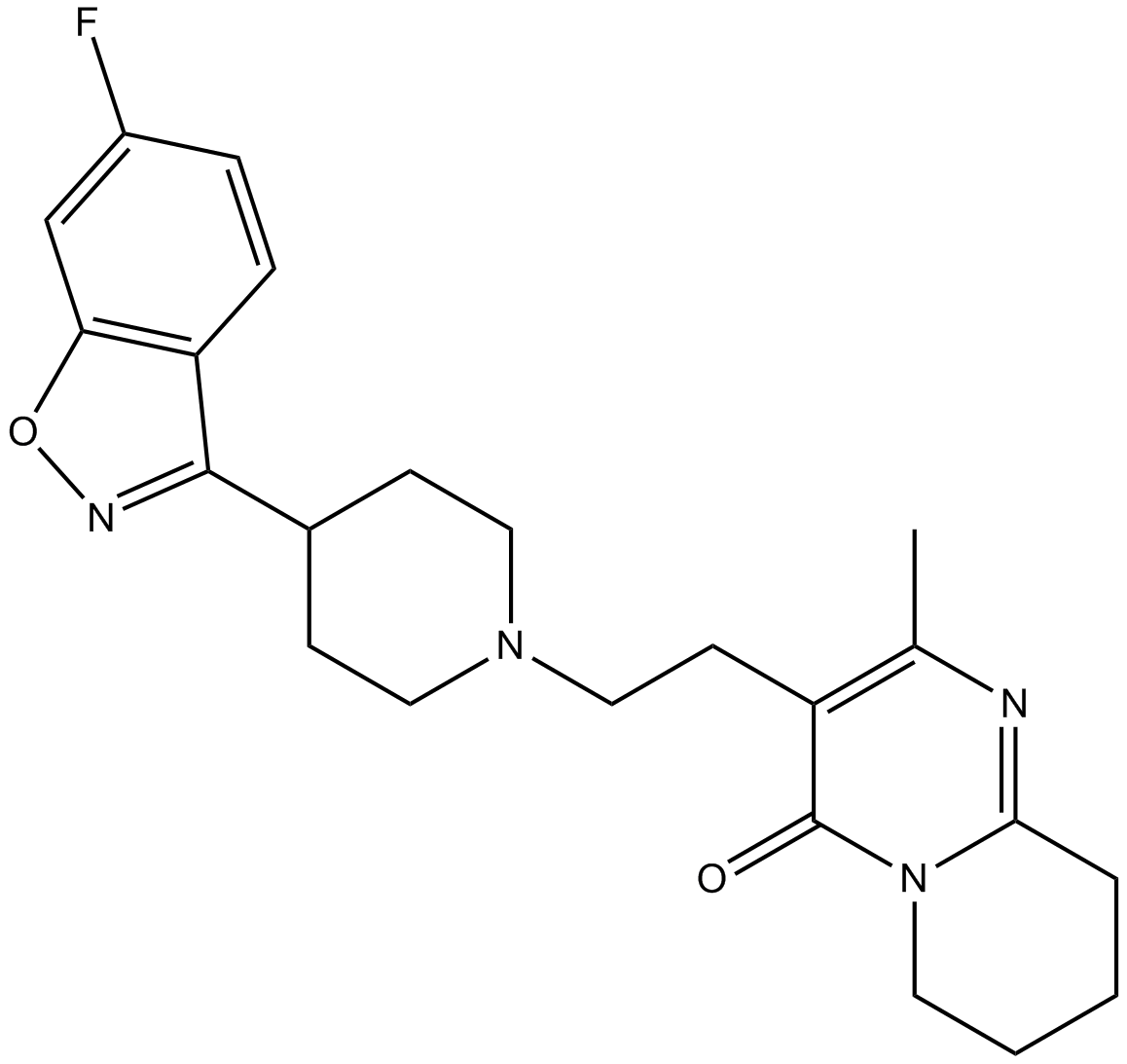
Cas No.: 106266-06-2
Sample solution is provided at 25 µL, 10mM.
Risperidone is a serotonin 5-HT2 receptor blocker, P-Glycoprotein inhibitor and potent dopamine D2 receptor antagonist, with Kis of 4.8, 5.9 nM for 5-HT2A and dopamine D2 receptor, respectively. Risperidone dose-dependently inhibited the release of IL-12 in mature DCs, while the production of IL-10 is dose-dependently increased by Risperidone. A high dose of risperidone can induce TNF-α release from mature DCs[3].
In the first experiment, body weight is found to be slightly but significantly lower in the Risperidone-treated rats as a function of age. Similar to the first experiment, age-dependent differences in body weight are also observed between the three treatment groups in the second locomotor experiment. Rats treated with the 3.0 mg/kg dose of Risperidone weigh less than vehicle-treated rats on postnatal days 35, 38, and 41. The third locomotor experiment involves larger, mixed-sex litters in contrast to the smaller, single-sex litters used in the first two experiments. As noted for the first two experiments, rats treated with Risperidone in the third experiment gain less weight in an age-dependent manner[4].
Reference:
[1]. Nyberg S, et al. 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology (Berl). 1993;110(3):265-72.
[2]. Chen ML, et al. Risperidone modulates the cytokine and chemokine release of dendritic cells and induces TNF-α-directed cell apoptosis in neutrophils. Int Immunopharmacol. 2012 Jan;12(1):197-204.
[3]. Zhu HJ, et al. Risperidone and paliperidone inhibit p-glycoprotein activity in vitro. Neuropsychopharmacology. 2007 Apr;32(4):757-64.
[4]. Bardgett ME, et al. Adult rats treated with risperidone during development are hyperactive. Exp Clin Psychopharmacol. 2013 Jun;21(3):259-67.
Average Rating: 5 (Based on Reviews and 28 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















