A 779 |
| رقم الكتالوجGC11004 |
A 779 هو مضاد محدد لمستقبل البروتين G المقترن (مستقبل Mas) ، وهو مستقبل Ang1-7 متميز عن AngII الكلاسيكي
Products are for research use only. Not for human use. We do not sell to patients.
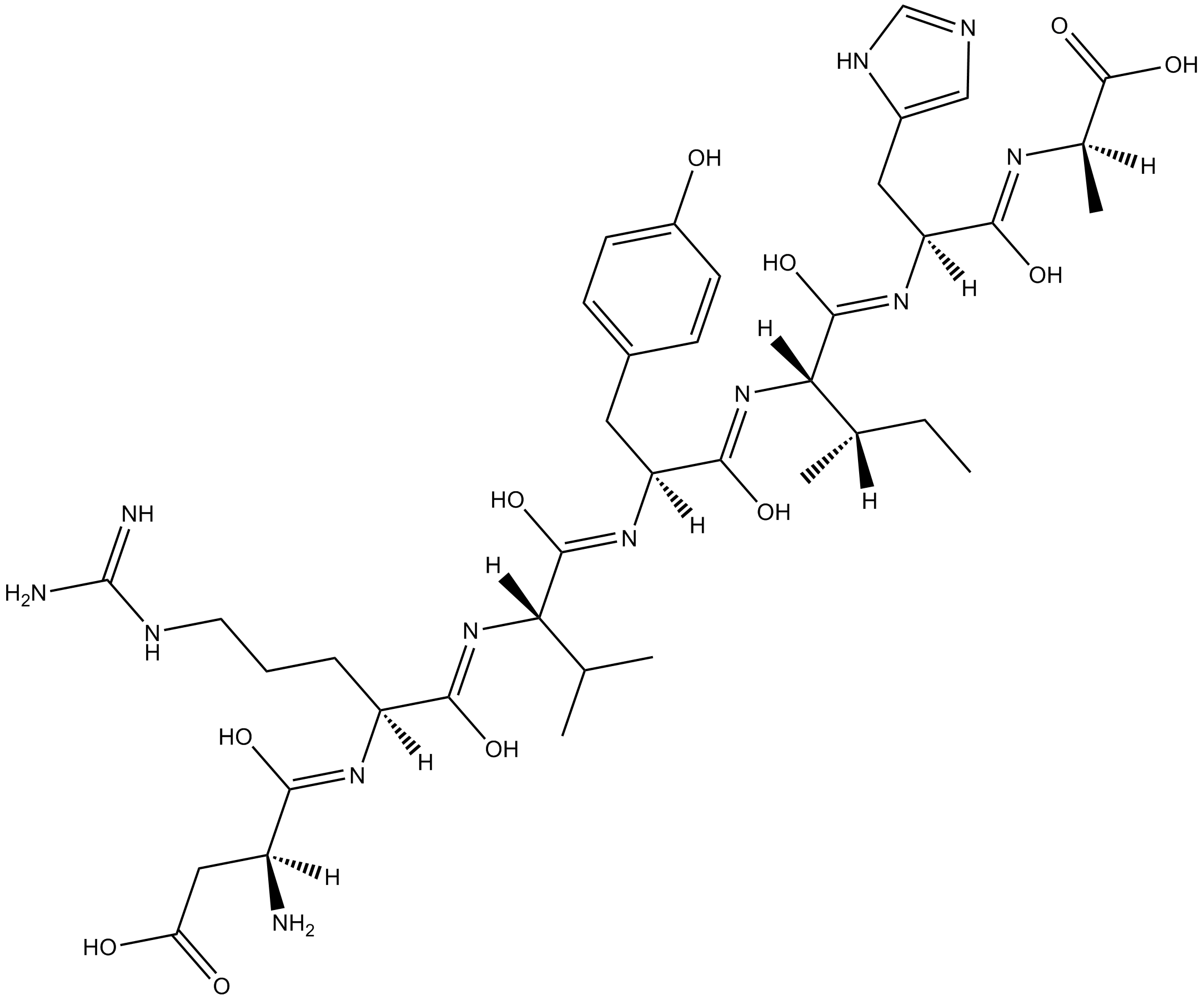
Cas No.: 159432-28-7
Sample solution is provided at 25 µL, 10mM.
A 779 is a potent and selective angiotensin-(1-7)/ Mas receptor antagonist [1].
Angiotensin-(1-7) is a bioactive component of the renin angiotensin system (RAS) which plays an important role in cardiovascular homeostasis and hydroelectrolyte balance in physiological and pathologic conditions [1][2].
A 779 is an angiotensin antagonist selective for the heptapeptide angiotensin-(I-7) [Ang-(l-7)] [1].
In water-loaded rats, A 779 significantly inhibited the antidiuretic effect of Ang-(l-7). In the rostral ventrolateral medulla, A-779 completely blocked the pressor effect produced by Ang-(1-7) microinjection [1]. In conscious Wistar rats, infusion of vehicle or A-779 (80 ng/ min, i.v.) started 40 to 45 minutes after captopril administration produced a significant shift of the BK dose-response curve obtained after captopril. A-779 significantly attenuated the potentiation of the hypotensive effect of BK by the ACE inhibitor captopril in conscious rats [2]. In anaesthetized male Wistar rats, when AT1R and AT2R were blocked, A779 increased renal blood flow/wet kidney tissue weight (RBF/KW). Co-blockade of all AT1R, AT2R, and MasR might alter RBF/ KW in male more than in female rats, which supported a crosstalk between MasR and Ang II receptors in renal circulation [3].
References:
Santos RA, Campagnole-Santos MJ, Baracho NC, et al. Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): evidence that the actions of angiotensin-(1-7) are mediated by specific angiotensin receptors. Brain Res Bull. 1994;35(4):293-8.
Maia LG, Ramos MC, Fernandes L, et al. Angiotensin-(1-7) antagonist A-779 attenuates the potentiation of bradykinin by captopril in rats. J Cardiovasc Pharmacol. 2004 May;43(5):685-91.
Mansoori A, Oryan S, Nematbakhsh M. Role of Mas receptor antagonist (A779) in renal hemodynamics in condition of blocked angiotensin II receptors in rats. Acta Physiol Hung. 2016 Mar;103(1):13-20.
Average Rating: 5 (Based on Reviews and 34 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















