Dorsomorphin (Compound C)
Dorsomorphin (DM) formerly known as compound C, is a potential AMPK (AMP-activated protein kinase) and bone morphogenetic protein (BMP) synthesis pathway inhibitor. Dorsomorphin competes with ATP for AMP when it is available in low amounts . Dorsomorphin (Compound C), according to research, suppresses tumour cell growth and triggers apoptosis via mechanisms that might be unrelated to the AMPK system. Also, in zebrafish embryos, it specifically inhibited the activity of the BMP type I receptors, which are ALK2, ALK3 and ALK6. In vitro along with a few in vivo biochemical investigations, compound C is frequently utilised. Most of its consumers are unlikely to be familiar with DM’s identification history. Scientists from Merck initially discovered this compound to be an AMPK inhibitor after screening a 10K library . They used a specific AMPK inhibitor primarily as a research instrument to outline the metformin mechanism of action and demonstrated selectivity vs. some unrelated kinases. Since its discovery, it has emerged as a crucial substance in the fields of molecular science and medicine, advancing the knowledge of cellular energy control and posing the possibility of therapeutic uses.
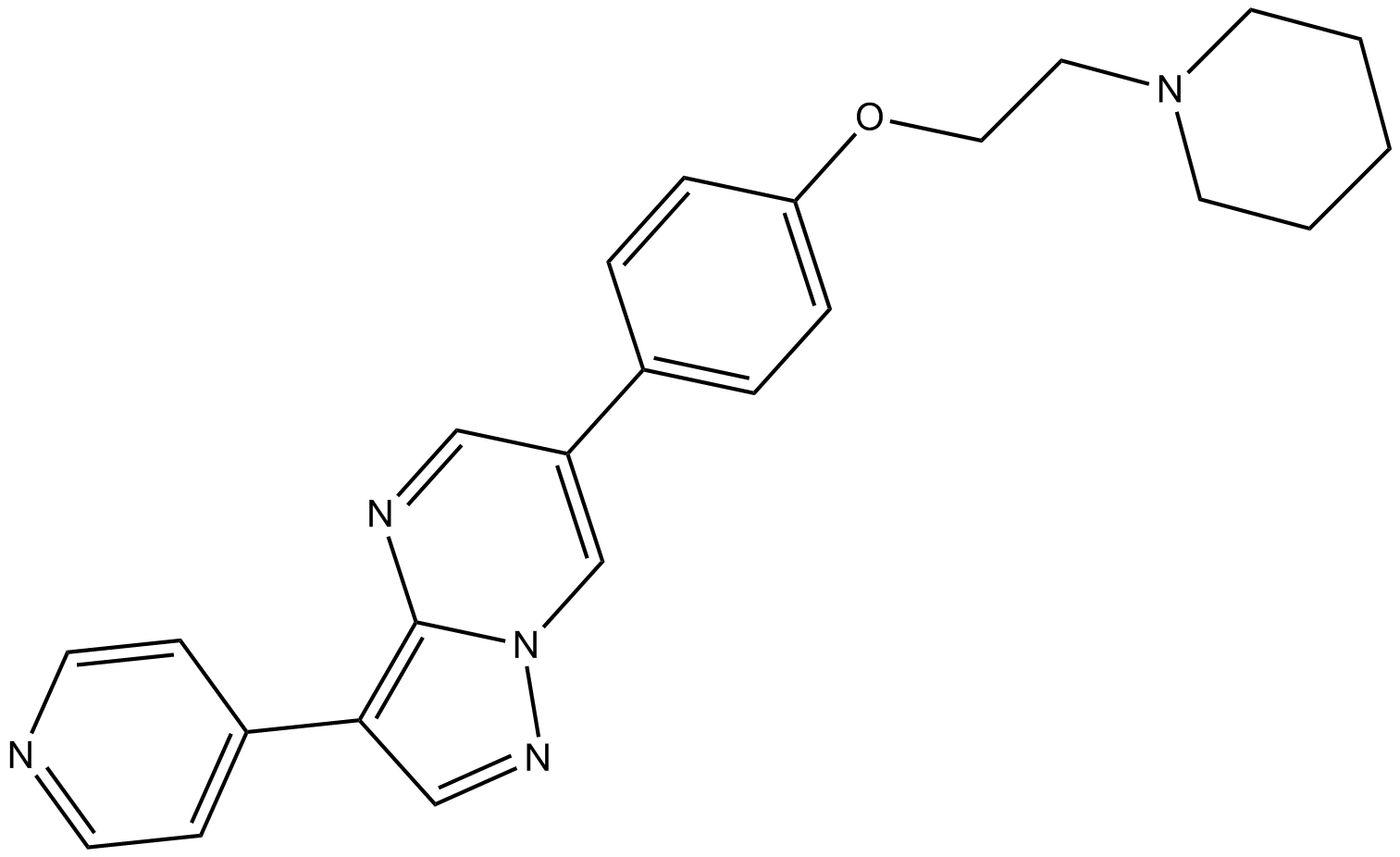
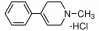
Dorsomorphin is a small molecule inhibitor with adaptable qualities and become both a useful tool for understanding numerous cellular mechanisms and a potential medical intervention. This little substance is selective, showing no noticeable inactivation of other types of kinases such as SYK, ZAPK, PKA and JAK3. The chemical name of Dorsomorphin is 6-[4-(2-piperidin-1-yl-ethoxy) phenyl]-3-pyridin-4-yl-pyrazolo [1, 5-a] pyrimidine and has a molecular formula of C24H25N5O, having molecular weight 399.5 g/mol. Dorsomorphin is a cell-permeable agent with an IC50 value of 234.6 nM for AMPK activity. The chemical structure of the compound is shown below (Figure 1).
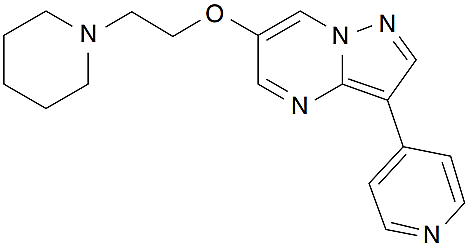
Figure 1: Chemical Structure of Compound C (Dorsomorphin);
AMPK acts as a molecular centre for cellular metabolic regulation and an essential energy sensor for cellular growth, expansion and metabolic control. It is a serine/threonine kinase and a heterotrimer comprising of α (catalytic), β and γ (regulatory) subunits. To provide vital nutrients and energy amid metabolic crisis, activated AMPK suppresses biosynthetic enzymes such as mTOR (required for protein synthesis) and acetyl CoA carboxylase (needed for lipid production). The high cellular ratio of AMP/ATP increases AMP binding to AMPK, causing phosphorylation and activation of α subunits, which must be specifically phosphorylated at Thr172 by downstream kinases, like CAMKKβ, LKB1 and others, for it to be fully activated. Studies have found a correlation between pharmacological stimulation of AMPK via two of its indirect activators (AICAR and metformin) and decreased proliferation of tumour cells . The effects of AICAR (activates the AMPK) and metformin on deactivating acetyl-CoA carboxylase (ACC) is inhibited by compound C substance. By decreased phosphorylation of the ACC, it was shown that Dorsomorphin has suppressed AMPK expression in a dose-dependent manner in several glioma cells. Also, the proliferation of the well-established glioma cell lines A172, T98G and U87 was potently reduced by this compound (Figure 2: A and B).
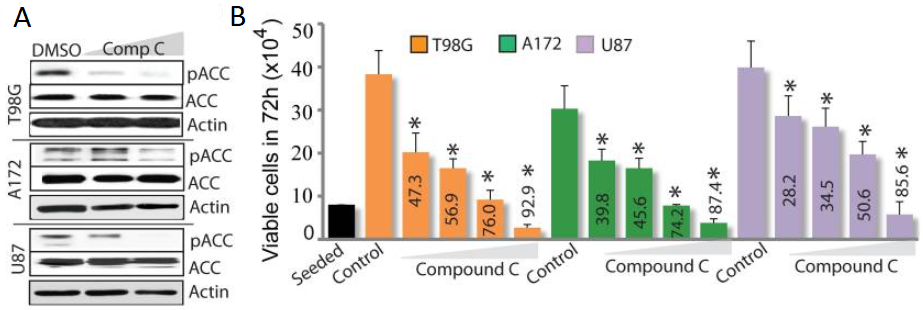
Figure 2: Immunoblots of three cell lines (T98G, A172 and U87)of glioma cells treated with compound C expressing pACC levels (A) and histogram depicting the dese-dependent effect of the same compound (at conc. 1, 2.5, 5 and 10μM) numbers within bars is the percentage of dead cells;
Moreover, to test whether Dorsomorphin provides an anti-glioma effect or not, the activity of AMPK was decreased genetically by specifically inhibiting it. The stability of the α subunits and formation of the AMPK complex depends on the β subunits. The proliferation test demonstrated that compound C (at concentrations of 5 and 10 μM) affects control and glioma cells (T98G) expressing 1 shRNA (Figure 3).
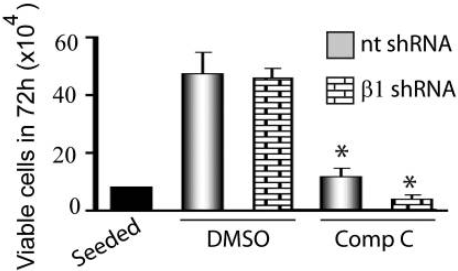
Figure 3: The effect of compound C on glioma cells (T98G) in control (nt) and β1 shRNA expressing cells.
It was found that compound C decreased proliferation irrespective of AMPK inhibition and that glioma cells silenced for AMPK were more susceptible to compound C's antiproliferative actions. This genetic information suggests that compound C does not decrease glioma growth through AMPK suppression. Surprisingly, compound C also serves as an effective inhibitor against the BMP pathway, even though its impact on cell proliferation is not well understood. However, it was discovered to block some other kinases besides AMPK.
Following multiple mechanisms, compound C prevents the proliferation of gliomas, for instance, the rapid growth of cancer cells, particularly gliomas, depends on signalling via the mTOR kinase pathway . Upon the activation of S6 kinase1 (S6K1) and 4EBP1 (translation initiation factor binding protein), the mTORC1 complex exerts its downstream effects, where S6, a ribosomal protein, is phosphorylated by S6K1. Compound C dramatically reduced S6 as well as 4EBP1 phosphorylation when applied to glioma cells, demonstrating its potency as an mTORC1 inhibitor (Figure 4).
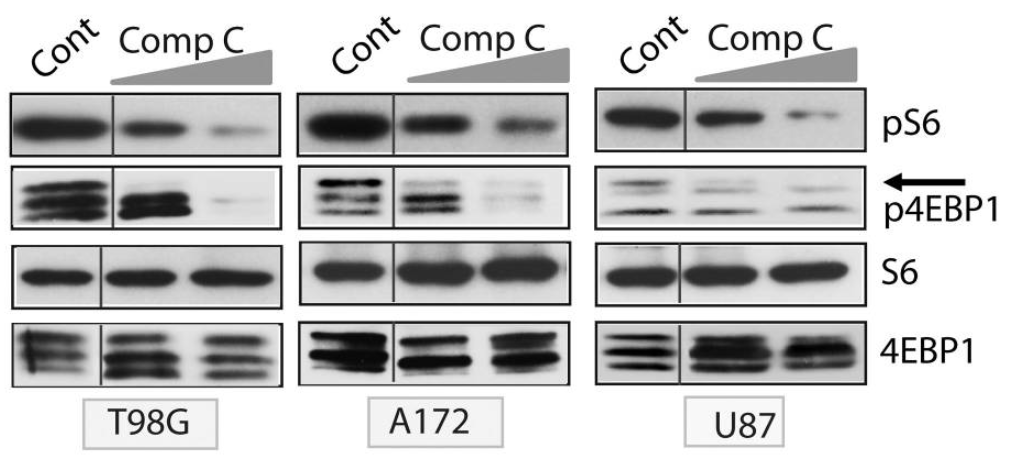
Figure 4: Immunoblots of glioma cells, treated with compound C showed significant inhibition of phosphorylation of S6 and 4EBP1;
The epithelial ovarian carcinoma (OvCa), specifically the HGSC (High-grade serous subtype), is detected at advanced stages with chemo-resistant relapse. It has been discovered that compound C inhibits OvCa invasiveness, proliferation and association with other cells, like macrophages and mesothelial cells, within the tumour microenvironment. The PI3K-AKT-NFκB pathways in these cancerous cells are inhibited as part of Dorsomorphin's mechanism of action . Also, compound C with cisplatin enhanced responsiveness towards experimental cisplatin treatment in animals and decreased tumour load in OvCa xenografts. Additionally, it re-sensitized cells taken from patients that were resistant to cisplatin.
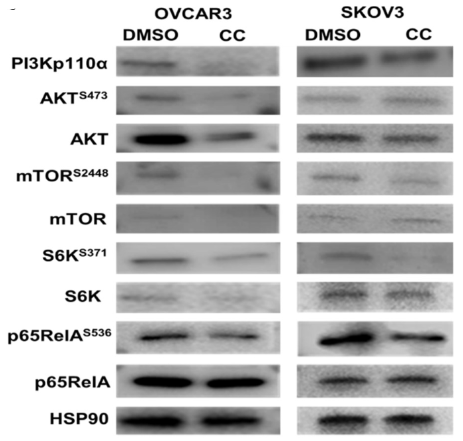
Figure 5: Western blot of SKOV3 and OVCAR3 cell lines depicting the effect of compound C on PI3K-AKT-mTOR-NFκB pathway while HSP90 is loaded as control.
Moreover, in another study by Li et al. it was demonstrated that Dorsomorphin affects the heat shock protein's (HSP) expression and apoptosis of cancer cells (in HeLa, HCT116 and PC3 cells). It was found to efficiently suppress the expression of HSPs, particularly HSP70, which are the proteins associated with cellular responses to stress and are frequently overproduced in cancer cells (Figure 5). Whereas programmed cell death was induced in cancer cells as a result of HSPs' suppression. This inhibition was caused by the inactivation of the HSF1, a transcription factor, normally involved in controlling the expression of HSPs. Dorsomorphin inhibited HSF1's translocation within the nucleus, where it might have triggered the formation of HSP. Further, both under regular circumstances as well as in heat stress, dorsomorphin decreased the concentrations of nuclear HSF1.
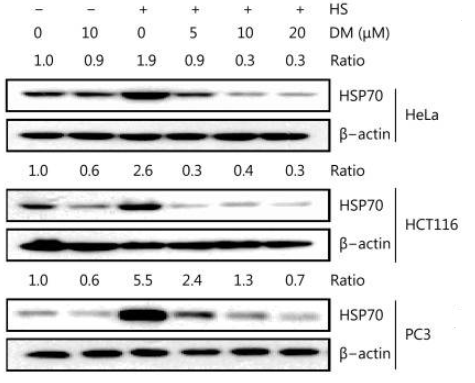
Figure 6: Western blot showing the protein expression of HSP70 in different cancer cells, treated with and without different concentrations of DM;
It was also demonstrated here that dorsomorphin's reduction of HSP expression was unrelated to the AMPK pathway and the ability to decrease the levels of HSP70 was unaffected by AMPK knockdown.













Comments