Dorsomorphin (Compound C) (Synonyms: Compound C) |
| Catalog No.GC17243 |
Dorsomorphin (Compound C) (Compound C) is a selective and ATP-competitive AMPK inhibitor (Ki=109 nM in the absence of AMP). Dorsomorphin (Compound C) (BML-275) selectively inhibits BMP type I receptors ALK2, ALK3, and ALK6. Dorsomorphin (Compound C) induces autophagy.
Products are for research use only. Not for human use. We do not sell to patients.
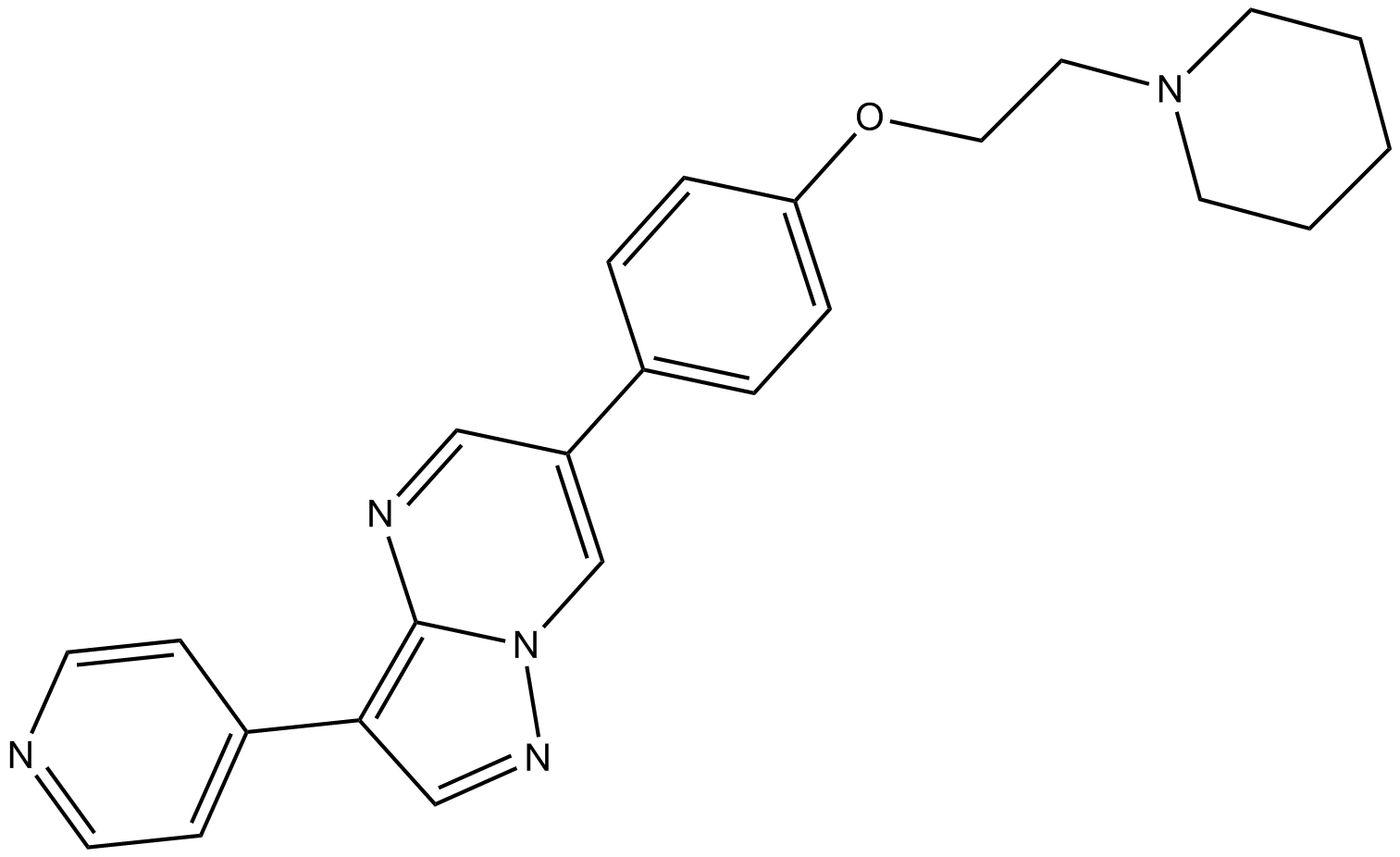
Cas No.: 866405-64-3
Sample solution is provided at 25 µL, 10mM.
Dorsomorphin (Compound C) is an agent that used as a cell-permeable AMPK inhibitor. It could rescue the antiproliferative actions of AICAR and metformin. Moreover, dorsomorphin (Compound C) is also used as a selective inhibitor of the BMP pathway. Compound C could inhibit a number of kinases other than AMPK.[1]
In vitro experiments indicate that Compound C inhibits AMPK activity and proliferation of human glioma cells. Dorsomorphin (Compound C) also reduces the apoptosis of cells induced by cisplatin, and decreases the expression of c-caspase3 and c-PARP in cisplatin treatment.
In vivo study demonstrate compound C attenuates cisplatin-induced nephrotoxicity in mice, and alleviates c-caspase 3 and c-PARP induced by cisplatin in kidney tissues.[1][2]
References:
[1].Liu X, et al. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther. 2014 Mar;13(3):596-605.
[2].Li F, et al. Compound C Protects Against Cisplatin-Induced Nephrotoxicity Through Pleiotropic Effects. Front Physiol. 2020 Dec 23;11:614244.
Average Rating: 5 (Based on Reviews and 11 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




