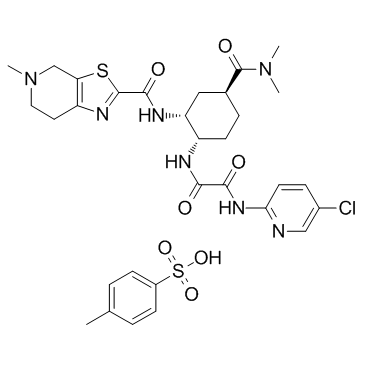
Edoxaban tosylate (Synonyms: DU-176b) |
| رقم الكتالوجGC35962 |
Edoxaban (DU-176b) tosylate هو مثبط عامل Xa (FXa) نشط عن طريق الفم وفعال للغاية وانتقائي ومباشر مع Kis 0.561 و 2.98 نانومتر للإنسان المجاني FXa والبروثرومبيناز.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 480449-71-6
Sample solution is provided at 25 µL, 10mM.
Edoxaban(DU-176) is an oral factor Xa (FXa) inhibitor in clinical development for stroke preventionIC50 Value:Target: factor XaEdoxaban is an oral factor Xa (FXa) inhibitor in clinical development for stroke prevention in patients with atrial fibrillation, an elderly population that frequently receives aspirin (ASA) and/or nonsteroidal anti-inflammatory drugs for concurrent illnesses[1].in vitro: Edoxaban PK was not affected by concomitant low-dose ASA or naproxen, but high-dose ASA increased systemic exposure of edoxaban by approximately 30%. The effects of edoxaban on prothrombin time, activated partial thromboplastin time, international normalized ratio, anti-FXa, and intrinsic FXa activity were not influenced by administration with ASA or naproxen. Inhibition of platelet aggregation by high-dose ASA, low-dose ASA, or naproxen was not affected by edoxaban[1].in vivo: Forty-eight subjects, aged 18 to 45 years, received either edoxaban 60 mg once daily × 7 days (n = 24) or digoxin 0.25 mg twice daily × 2 days and once daily × 5 days (n = 24) and then concomitantly for 7 days. Serial blood and urine samples were collected for digoxin and edoxaban concentrations on days 7 and 14. Serial coagulation assays were measured for edoxaban on days 7 and 14. Edoxaban PK parameters demonstrated mild increases in area under the curve and peak concentrations of 9.5% and 15.6%, respectively[2],Clinical trial: Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans was reported[3].
[1]. Mendell J, Lee F, Chen S, The Effects of the Antiplatelet Agents, Aspirin and Naproxen, on Pharmacokinetics and Pharmacodynamics of the Anticoagulant Edoxaban, a Direct Factor Xa Inhibitor. J Cardiovasc Pharmacol. 2013 Apr 23. [Epub ahead of print] [2]. Mendell J, Noveck RJ, Shi M. Pharmacokinetics of the direct factor Xa inhibitor edoxaban and digoxin administered alone and in combination. J Cardiovasc Pharmacol. 2012 Oct;60(4):335-41. [3]. Bathala MS, Masumoto H, Oguma T, Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012 Dec;40(12):2250-5.
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















