Edoxaban tosylate (Synonyms: DU-176b) |
| Catalog No.GC35962 |
Le tosylate d'edoxaban (DU-176b) est un inhibiteur du facteur Xa (FXa) actif par voie orale, très puissant, sélectif et direct avec Kis de 0,561 et 2,98 nM pour le FXa humain libre et la prothrombinase.
Products are for research use only. Not for human use. We do not sell to patients.
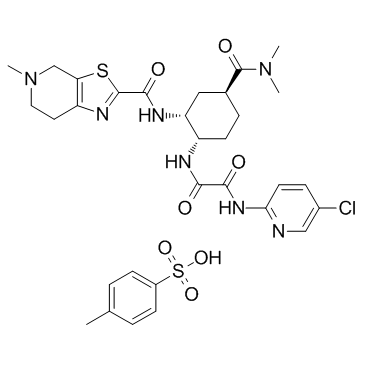
Cas No.: 480449-71-6
Sample solution is provided at 25 µL, 10mM.
Edoxaban(DU-176) is an oral factor Xa (FXa) inhibitor in clinical development for stroke preventionIC50 Value:Target: factor XaEdoxaban is an oral factor Xa (FXa) inhibitor in clinical development for stroke prevention in patients with atrial fibrillation, an elderly population that frequently receives aspirin (ASA) and/or nonsteroidal anti-inflammatory drugs for concurrent illnesses[1].in vitro: Edoxaban PK was not affected by concomitant low-dose ASA or naproxen, but high-dose ASA increased systemic exposure of edoxaban by approximately 30%. The effects of edoxaban on prothrombin time, activated partial thromboplastin time, international normalized ratio, anti-FXa, and intrinsic FXa activity were not influenced by administration with ASA or naproxen. Inhibition of platelet aggregation by high-dose ASA, low-dose ASA, or naproxen was not affected by edoxaban[1].in vivo: Forty-eight subjects, aged 18 to 45 years, received either edoxaban 60 mg once daily × 7 days (n = 24) or digoxin 0.25 mg twice daily × 2 days and once daily × 5 days (n = 24) and then concomitantly for 7 days. Serial blood and urine samples were collected for digoxin and edoxaban concentrations on days 7 and 14. Serial coagulation assays were measured for edoxaban on days 7 and 14. Edoxaban PK parameters demonstrated mild increases in area under the curve and peak concentrations of 9.5% and 15.6%, respectively[2],Clinical trial: Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans was reported[3].
[1]. Mendell J, Lee F, Chen S, The Effects of the Antiplatelet Agents, Aspirin and Naproxen, on Pharmacokinetics and Pharmacodynamics of the Anticoagulant Edoxaban, a Direct Factor Xa Inhibitor. J Cardiovasc Pharmacol. 2013 Apr 23. [Epub ahead of print] [2]. Mendell J, Noveck RJ, Shi M. Pharmacokinetics of the direct factor Xa inhibitor edoxaban and digoxin administered alone and in combination. J Cardiovasc Pharmacol. 2012 Oct;60(4):335-41. [3]. Bathala MS, Masumoto H, Oguma T, Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012 Dec;40(12):2250-5.
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















