Glesatinib hydrochloride (Synonyms: MGCD265 hydrochloride) |
| رقم الكتالوجGC19166 |
هيدروكلوريد جليساتينيب (MGCD265 هيدروكلوريد) هو مثبط ثنائي فعال عن طريق الفم وقوي MET / SMOجليساتينيب هيدروكلوريد ، مثبط التيروزين كيناز ، يعاكس مقاومة الأدوية المتعددة (MDR) في سرطان الرئة ذو الخلايا غير الصغيرة (NSCLC)
Products are for research use only. Not for human use. We do not sell to patients.
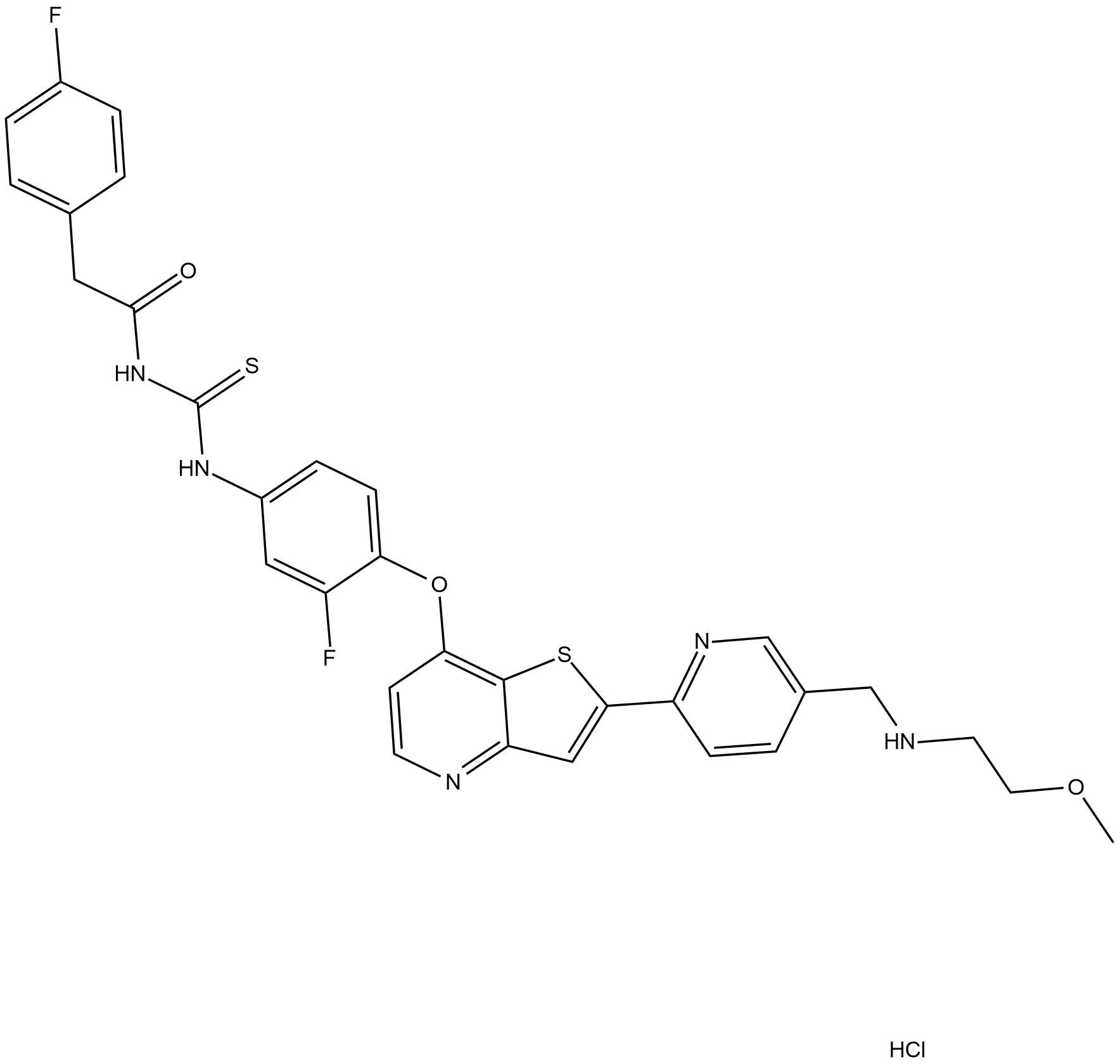
Cas No.: 1123838-51-6
Sample solution is provided at 25 µL, 10mM.
Glesatinib hydrochloride is an inhibitor of the MET and Axl receptor tyrosine kinase pathways, which drive tumour growth when altered.Target: MET, AxlGlesatinib is an orally bioavailable, small-molecule, multitargeted tyrosine kinase inhibitor with potential antineoplastic activity. MGCD265 binds to and inhibits the phosphorylation of several receptor tyrosine kinases (RTKs), including the c-Met receptor (hepatocyte growth factor receptor); the Tek/Tie-2 receptor; vascular endothelial growth factor receptor (VEGFR) types 1, 2, and 3; and the macrophage-stimulating 1 receptor (MST1R or RON). Glesatinib is a tyrosine kinase inhibitor that is expected to potently and selectively target tumors in patients with driver alterations in MET (mutations and gene amplification) and Axl (rearrangements) that occur in approximately 8% of patients with non-small cell lung cancer (NSCLC). Glesatinib is being evaluated in a Phase 1b study in patients with solid tumors that have genetic alterations in MET or AXL genes. The Phase 2 trial in NSCLC patients with MET genetic alterations is underway to confirm and extend the data that supports the clinical benefit of Glesatinib in patients with driver mutations in MET. Genetic alterations in these targets have been implicated as drivers of tumor growth and disease progression in NSCLC, gastroesophageal cancer and other solid tumors. MET and Axl are also implicated as drivers of tumor progression in patients whose tumors have become resistant to EGFR inhibitors. Therefore, Mirati believes that the combination of Glesatinib with an EGFR inhibitor could potentially treat patients who have become resistant to agents targeting EGFR. Mirati retains worldwide rights to Glesatinib.
Average Rating: 5 (Based on Reviews and 29 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















