Panobinostat (LBH589) (Synonyms: LBH589) |
| رقم الكتالوجGC12257 |
A pan-HDAC inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
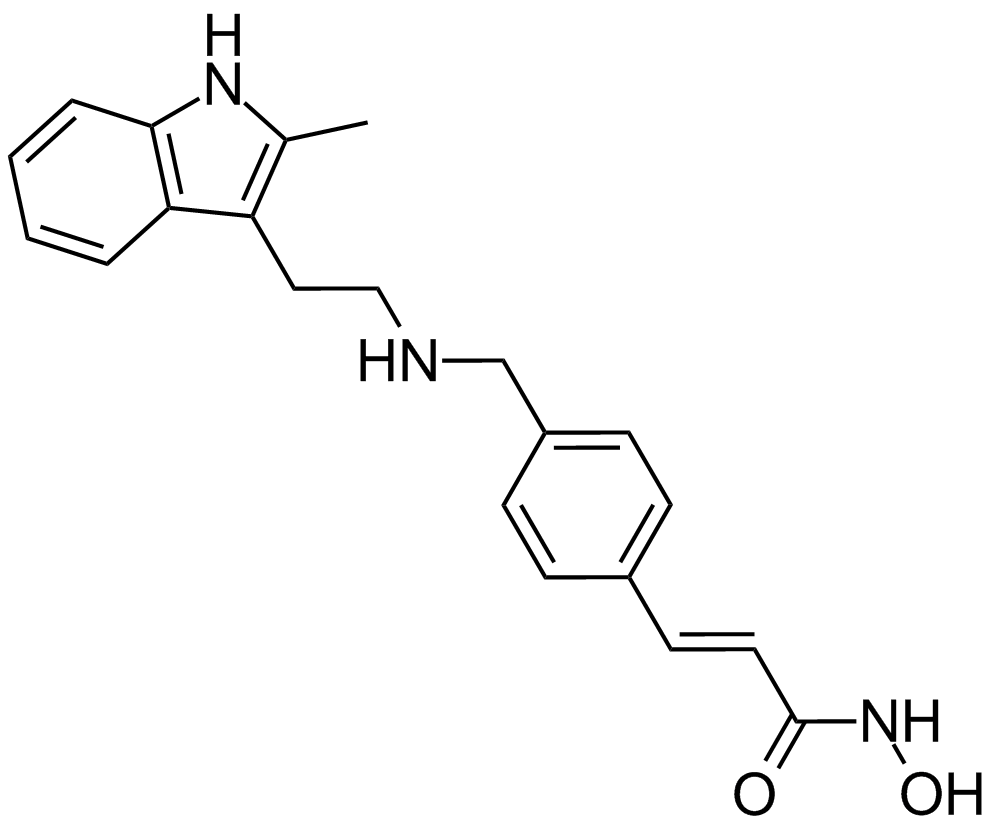
Cas No.: 404950-80-7
Sample solution is provided at 25 µL, 10mM.
Panobinostat, as known as LBH589, is a novel and potent hydroxamic acid-based deacetylase inhibitor (DACis)that inhibits a broad spectrum of histone deacetylases (HDACs), including all Classes 1, 2 and 4 HDAC enzymes, at low nanomolar concentrations. According to previous studies, it not only induces apoptosis in multiple myeloma (MM) cells via caspase activation and poly(ADP-ribose) polymerase (PARP) cleavage, but also induces potent cell growth inhibition, cell-cycle arrest, and apoptosis in a time- and dose-dependent manner in both Philadelphia chromosome-negative (Ph-) actue lymphoblastic leukemia (ALL) cells lines (T-cell MOLT-4 and pre-B-cell Reh), which are correlated with induction of histone (H3K9 and H4K8) hyperacetylation, activation of p21 and p27, and suppression of c-Myc.
Reference
[1].Wenlin Shao, Joseph D. Growney, Yun Feng, Gregory O’Connor, Minying Pu, Wenjing Zhu, Yung-Mae Yao, Paul Kwon, Stephen Fawell and Peter Atadja. Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: defining molecular mechanisms of resistance. Int. J. Cancer: 127, 2199-2208 (2010)
[2].Laurence Catley, Ellen Weisberg, Tanyel Kiziltepe, Yu-Tzu Tai, Teru Hideshima, Paola Neri, Pierfrancesco Tassone, Peter Atadja, Dharminder Chauhan, Nikhil C. Munshi and Keneth C. Anderson. Aggresome induction by proteasome inhibitor bortezomib and α-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood (2006); 108(10): 3441-3449
[3].Anna Scuto, Mark Kirschbaum, Claudia Kowolik, Leo Kretzner, Agnes Juhasz, Peter Atadja, Vinod Pullarkat, Ravi Bhatia, Stephen Forman, Yun Yen, and Richard Jove. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood (2008); 111(10):5093-5100
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















