Acebilustat (Synonyms: CTX-4430) |
| Catalog No.GC19018 |
El acebilustato (CTX-4430) es un inhibidor de la hidrolasa de leucotrieno A4, utilizado como fÁrmaco antiinflamatorio oral.
Products are for research use only. Not for human use. We do not sell to patients.
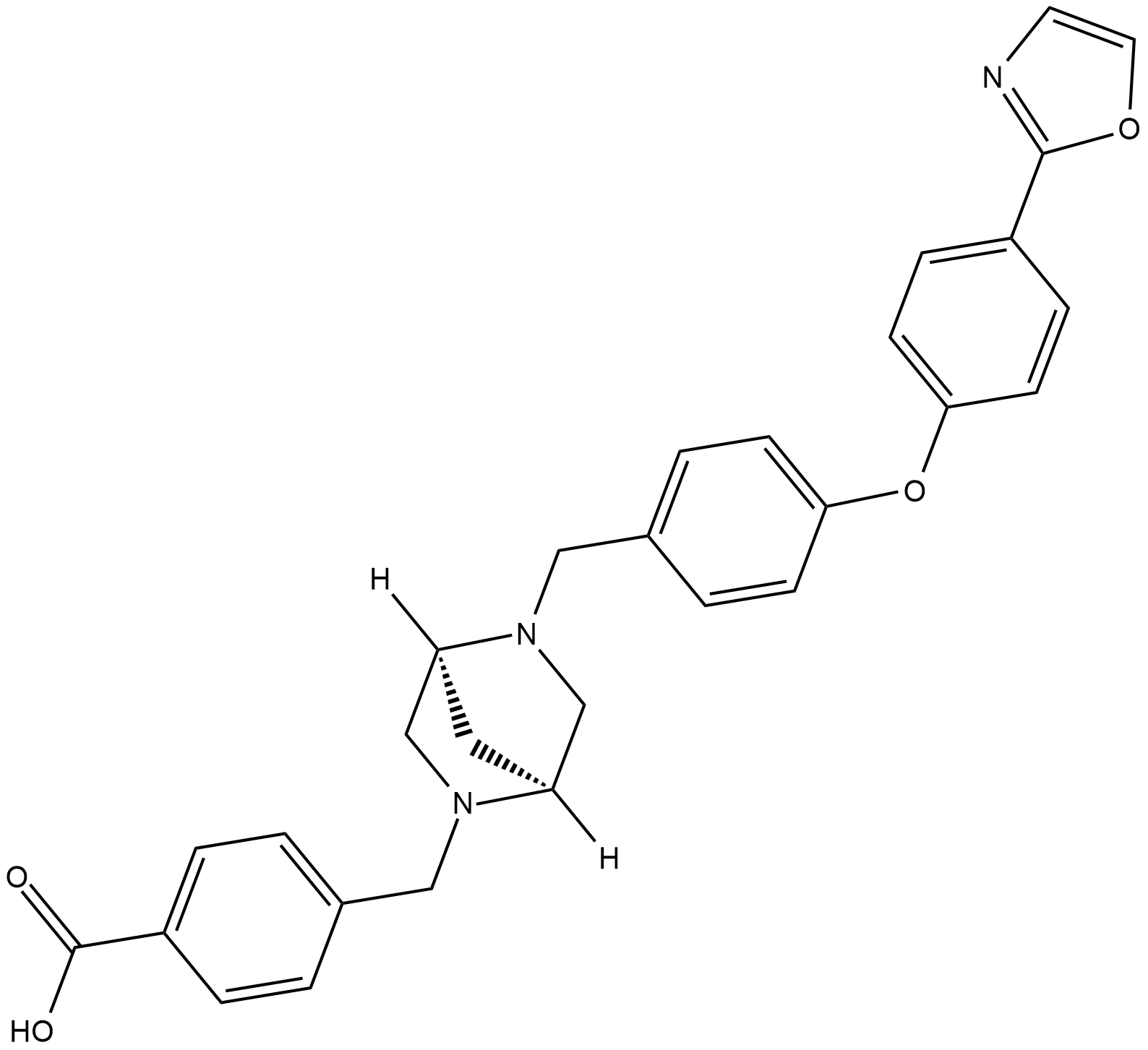
Cas No.: 943764-99-6
Sample solution is provided at 25 µL, 10mM.
Acebilustat is a leukotriene A4 hydrolase inhibitor, used for an oral antiinflammatory drug.
Acebilustat is a leukotriene A4 hydrolase inhibitor, which is safe and well tolerated in phase 1 trial[1]. Acebilustat is an antiinflammatory drug in development for the treatment of CF and other diseases. It is a potent inhibitor of the enzyme leukotriene A4 hydrolase (LTA4H), which catalyzes the rate-limiting step in the formation of leukotriene B4 (LTB4), a potent chemoattractant and activator of inflammatory immune cells including neutrophils[2].
References:
[1]. Elborn JS, et al. Phase 1 Studies of Acebilustat: Biomarker Response and Safety in Patients with Cystic Fibrosis. Clin Transl Sci. 2016 Nov 2.
[2]. Elborn JS, et al. Phase I Studies of Acebilustat: Pharmacokinetics, Pharmacodynamics, Food Effect, and CYP3A Induction. Clin Transl Sci. 2016 Oct 28.
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















