Alfuzosin-d7 (Synonyms: SL 77499-10-d7, Uroxatral-d7) |
| Catalog No.GC52023 |
An internal standard for the quantification of alfuzosin
Products are for research use only. Not for human use. We do not sell to patients.
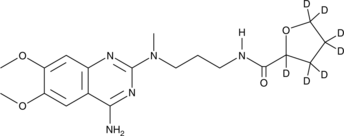
Cas No.: 1133386-93-2
Sample solution is provided at 25 µL, 10mM.
Alfuzosin-d7 is intended for use as an internal standard for the quantification of alfuzosin by GC- or LC-MS. Alfuzosin is an antagonist of α1-adrenergic receptors (α1-ARs; Kis = 10, 10, and 3.16 nM for α1A-, α1B-, and α1D-ARs, respectively).1 It increases the amount of phenylephrine needed to increase urethral and arterial blood pressure in pithed rats when administered at a dose of 10 mg/kg.2 Formulations containing alfuzosin have been used in the treatment of benign prostatic hyperplasia.
1.Kenny, B.A., Miller, A.M., Williamson, I.J.R., et al.Evaluation of the pharmacological selectivity profile of α1 adrenoceptor antagonists at prostatic α1 adrenoceptors: Binding, functional and in vivo studiesBr. J. Pharmacol.118(4)871-878(1996) 2.Martin, D.J., Lluel, P., Pouyet, T., et al.Relationship between the effects of alfuzosin on rat urethral and blood pressures and its tissue concentrationsLife Sci.63(3)169-176(1998)
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















