Azidoindolene 1 |
| Catalog No.GC42892 |
UR-144 and XLR11 are potent synthetic cannabinoids (CBs) that have been identified as adulterants of herbal products.
Products are for research use only. Not for human use. We do not sell to patients.
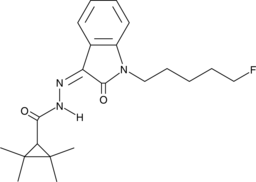
Cas No.: 1364933-69-6
Sample solution is provided at 25 µL, 10mM.
UR-144 and XLR11 are potent synthetic cannabinoids (CBs) that have been identified as adulterants of herbal products.[1],[2] Azidoindolene 1 is structurally similar to UR-144, XLR11, and a number of additional synthetic CBs with a hydrazide linking the tetramethylcyclopropyl group to the aminoalkylindole group. The physiological and toxicological properties of this compound are not known. Typically, a tetramethylcyclopropyl group confers selectivity for the peripheral CB2 receptor, and the addition of substituents at the N1-amine of the aminoalkylindole group is necessary for high affinity at either CB1 or CB2.[3] This product is intended for forensic and research applications.
Reference:
[1]. Uchiyama, N., Kawamura, M., Kikura-Hanajiri, R., et al. URB-754: A new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Science International 227(1-3), 21-32 (2013).
[2]. Uchiyama, N., Matsuda, S., Kawamura, M., et al. Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol. 31(2), 223-240 (2013).
[3]. Frost, J.M., Dart, M.J., Tietje, K.R., et al. Indol-3-ylcycloalkyl ketones: Effects of N1 substituted indole side chain variations on CB2 cannabinoid receptor activity. J. Med. Chem. 53(1), 295-315 (2010).
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















