Fezolinetant (ESN-364) |
| Catalog No.GC30104 |
Fezolinetant (ESN-364) es un antagonista del receptor de neuroquinina 3 (NK3R), utilizado para el tratamiento de los sofocos menopÁusicos.
Products are for research use only. Not for human use. We do not sell to patients.
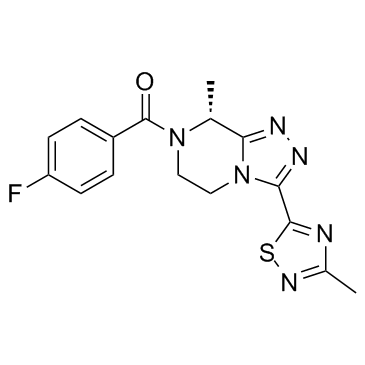
Cas No.: 1629229-37-3
Sample solution is provided at 25 µL, 10mM.
Fezolinetant is an antagonist of the neurokinin 3 receptor (NK3R), used for the treatment of menopausal hot flushes.
Fezolinetant (ESN364, 1 mg/kg, iv bolus) reversibly inhibits the regular, pulsatile secretion of LH in the ovarectomized ewe. ESN364 represses the pulse pattern of LH in all treated animals. ESN364 (5 mg/kg, p.o.) lowers plasma LH, but not FSH, in the castrated monkey. ESN364 (10, 25, 50 mg/kg, orally) also blocks the LH surge and decreases ovarian hormone levels throughout the menstrual cycle in monkeys[1].
[1]. Fraser GL, et al. The NK3 Receptor Antagonist ESN364 Interrupts Pulsatile LH Secretion and Moderates Levels of Ovarian Hormones Throughout the Menstrual Cycle. Endocrinology. 2015 Nov;156(11):4214-25.
Average Rating: 5 (Based on Reviews and 11 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















