S116836 |
| Catalog No.GC63332 |
S116836, un potente inhibidor de la tirosina quinasa BCR-ABL activo por vÍa oral, bloquea tanto la Bcr-Abl de tipo salvaje como la T315I. S116836 detiene las células en la fase G0/G1 del ciclo celular, induce la apoptosis, aumenta la producciÓn de ROS y disminuye la producciÓn de GSH en las células BaF3/WT y BaF3/T315I. S116836 también inhibe SRC, LYN, HCK, LCK y BLK, y tirosina quinasas receptoras como FLT3, TIE2, KIT, PDGFR-β. Actividades antitumorales.
Products are for research use only. Not for human use. We do not sell to patients.
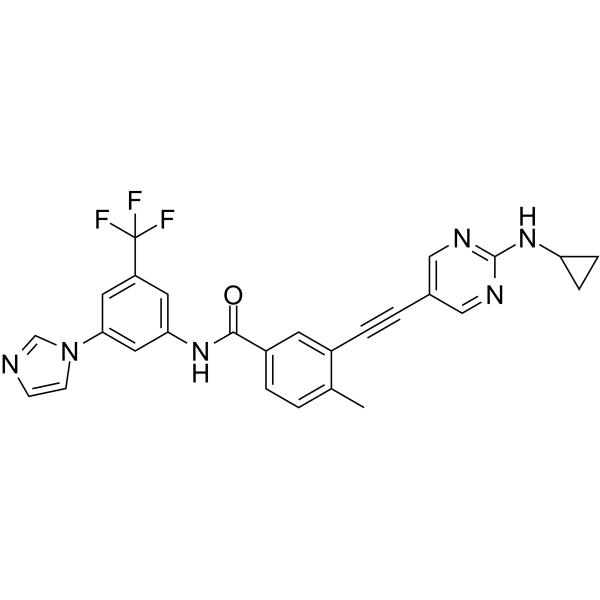
Cas No.: 1257628-57-1
Sample solution is provided at 25 µL, 10mM.
S116836, a potent, orally active BCR-ABL tyrosine kinase inhibitor (TKI), blocks both wild-type as well as T315I Bcr-Abl. S116836 potently inhibits the phosphorylation of BCR-ABL and induces apoptosis. S116836 inhibits growth of WT and T315I mutant BCR-ABL tumors and does not cause significant cardiotoxicity. S116836 also inhibits SRC, LYN, HCK, LCK and BLK, and receptor tyrosine kinases such as FLT3, TIE2, KIT, PDGFR-β. Antitumor activies[1][2][3].
S116836 (0.01-1 μM; 24 hours) significantly reduces the cellular proliferation of BaF3/WT and BaF3/T315I cells (IC50 values of 0.05 μM and 0.20 μM, respectively)[1].S116836 (0.01-1 μM; 24 hours) significantly downregulates the expression level of p-BCR-ABL in BaF3/WT cells[1].S116836 (0.01-1 μM; 24 hours) significantly downregulates the expression level of p-BCR-ABL in Imatinib-resistant BaF3/T315I cells[1].S116836 (0.01-1 μM; 24 hours) significantly downregulates the expression level of p-Crkl and p-STAT5 (downstream signaling proteins of BCR-ABL) in both BaF3/WT and BaF3/T315I cells[1].S116836 (0.1, 0.3, and 0.5 μM; 24 hours) arrests the BaF3/WT and BaF3/T315I cells in G0/G1 phase of the cell cycle[1].S116836 (0.3 and 0.5 μM; 24 hours) increases ROS production and decreases GSH levels in BaF3/WT and BaF3/T315I cells[1].S116836 (0.1, 0.3, and 0.5 μM; 24 hours) induces apoptosis in BaF3/WT and BaF3/T315I cells[1].SAHA and S116836 synergistically induce apoptosis in imatinib-resistant chronic myelogenous leukemia cells[2].S116836 potently inhibits PDGFRα and its downstream signaling molecules such as STAT3, AKT, and Erk1/2. S116836 effectively inhibits the growth of the WT and T674I FIP1L1-PDGFRα-expressing neoplastic cells. S116836 induces intrinsic pathway of apoptosis as well as the death receptor pathway, coincided with up-regulation of the proapoptotic BH3-only protein Bim-EL through the Erk1/2 pathway[3].
S116836 (100 or 200 mg/kg; i.p.; q3d×6, athymic NCR nude mice) decreases the volume and weight of xenograft tumors expressing WT and T315I mutant BCR-ABL[1].S116836 (200mg/kg/d, oral gavage for 14 days) inhibits the growth of xenografted T674I-FIP1L1-PDGFRα cells in nude mice[3].
[1]. Gupta P, et al. Preclinical development of a novel BCR-ABL T315I inhibitor against chronic myeloid leukemia. Cancer Lett. 2020;472:132-141. [2]. Bu Q, et al. SAHA and S116836, a novel tyrosine kinase inhibitor, synergistically induce apoptosis in imatinib-resistant chronic myelogenous leukemia cells. Cancer Biol Ther. 2014;15(7):951-962. [3]. Shen Y, et al. Antitumor activity of S116836, a novel tyrosine kinase inhibitor, against imatinib-resistant FIP1L1-PDGFRα-expressing cells. Oncotarget. 2014;5(21):10407-10420.
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















