Sorafenib Tosylate (Synonyms: Bay 43-9006 Tosylate) |
| Catalog No.GC16499 |
A multi-kinase inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
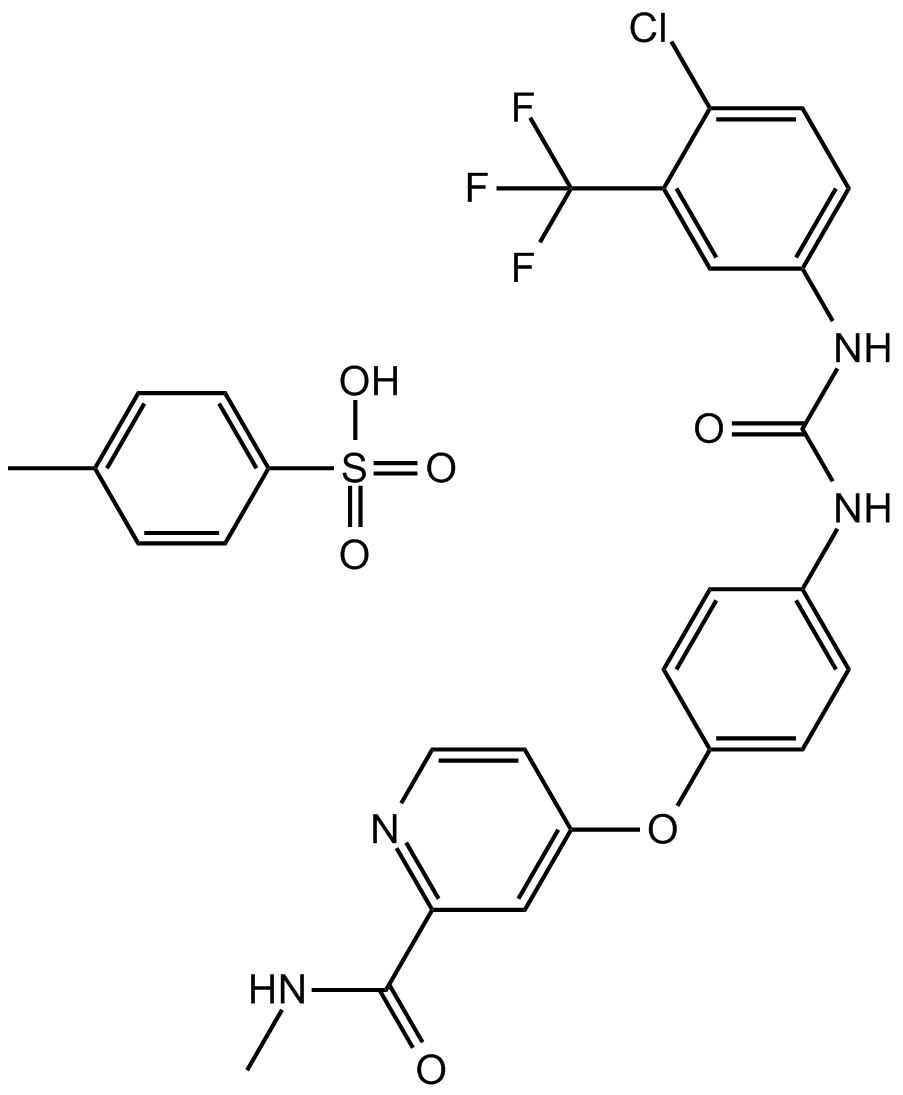
Cas No.: 475207-59-1
Sample solution is provided at 25 µL, 10mM.
Sorafenib tosylate, also named nexavar, is a small-molecule anticancer compound [1]. It is also a novel oral Raf kinase and a vascular endothelial growth factor receptor (VEGFR) inhibitor. It inhibits tumor cell proliferation and tumor angiogenesis [2]. To HepG2 cells (1× 106), the IC50 of sorafenib tosylate is 2.09μg/ml [3].
Raf is a mitogen-stimulated protein kinase that functions as a component of the signaling cascade that leads to the stimulation of mitogen-activated protein kinase [4].
Vascular endothelial growth factor (VEGF) is a highly specific mitogen for vascular endothelial cells [5].
Treatment with nexavar potently inhibited the cell proliferation of MV4-11 cells (FLT3-ITD) in a dose-dependent manner with an IC50 of 0.88 nM. In MV4-11 cells, sorafenib tosylate of a concentration of 100 nM induced 43.6±5.2% of the cells to undergo apoptosis whereas in EOL-1 cells a concentration as low as 10 nM induced 89.29±1.8% of the cells to be apoptotic [6].
Nude rats at the age of 6 weeks injected with 105 MDA-MB-231 cells were involved. After monotherapy with sorafenib tosylate a significant reduction of the osteolytic lesion volume was observed on days 45 and 55 and of the soft tissue component volume on day 55 in comparison to untreated animals (p < 0.05). Compared to controls, treatment with sorafenib tosylate made bone metastases show significantly decreased values of Amplitude A and kep from day 35 to 55 (Amplitude A: p<0.01; kep p<0.01 on days 35 and 55; p<0.05 on day 45) [7].
References:
[1]. Chetan Lathia, John Lettieri, Frank Cihon, et al. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol, 2006, 57: 685-692.
[2]. M. J. Gnoth, S. Sandmann, K. Engel, et al. In Vitro to In Vivo Comparison of the Substrate Characteristics of Sorafenib Tosylate toward P-Glycoprotein. Drug Metabolism & Disposition, 2010, 38: 1341–1346.
[3]. Sayantan Dey, Subhadeep Roy, Nilanjana Deb, et al. Anti-carcinogenic Activity of Ruellia Tuberosa L. (Acanthaceae) Leaf Extract on Hepatoma Cell Line & Increased Superoxide Dismutase Activity on Macrophage Cell Lysate. Int J Pharm Pharm Sci, 2010, 5(Suppl 3): 854-861.
[4]. Markus Wartmann and Roger J. Davis. The Native Structure of the Activated Raf Protein Kinase Is a Membrane-bound Multi-subunit Complex. The Journal of Biological Chemistry, 1994, 269(9): 6695-6701.
[5]. Gera Neufeld, Tzafra Cohen, Stela Gengrinovitch, et al. Vascular endothelial growth factor (VEGF) and its receptors. The FASEB Journal, 1999, 13: 9-22.
[6]. D Auclair, D Miller, V Yatsula, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia, 2007, 21:439-445.
[7]. Maximilian Merz, Dorde Komljenovic, Stefan Zwick, et al. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. European Journal of Cancer, 2011, 47:277-286.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




