TAS-102 (Synonyms: TAS-102) |
| Catalog No.GC19348 |
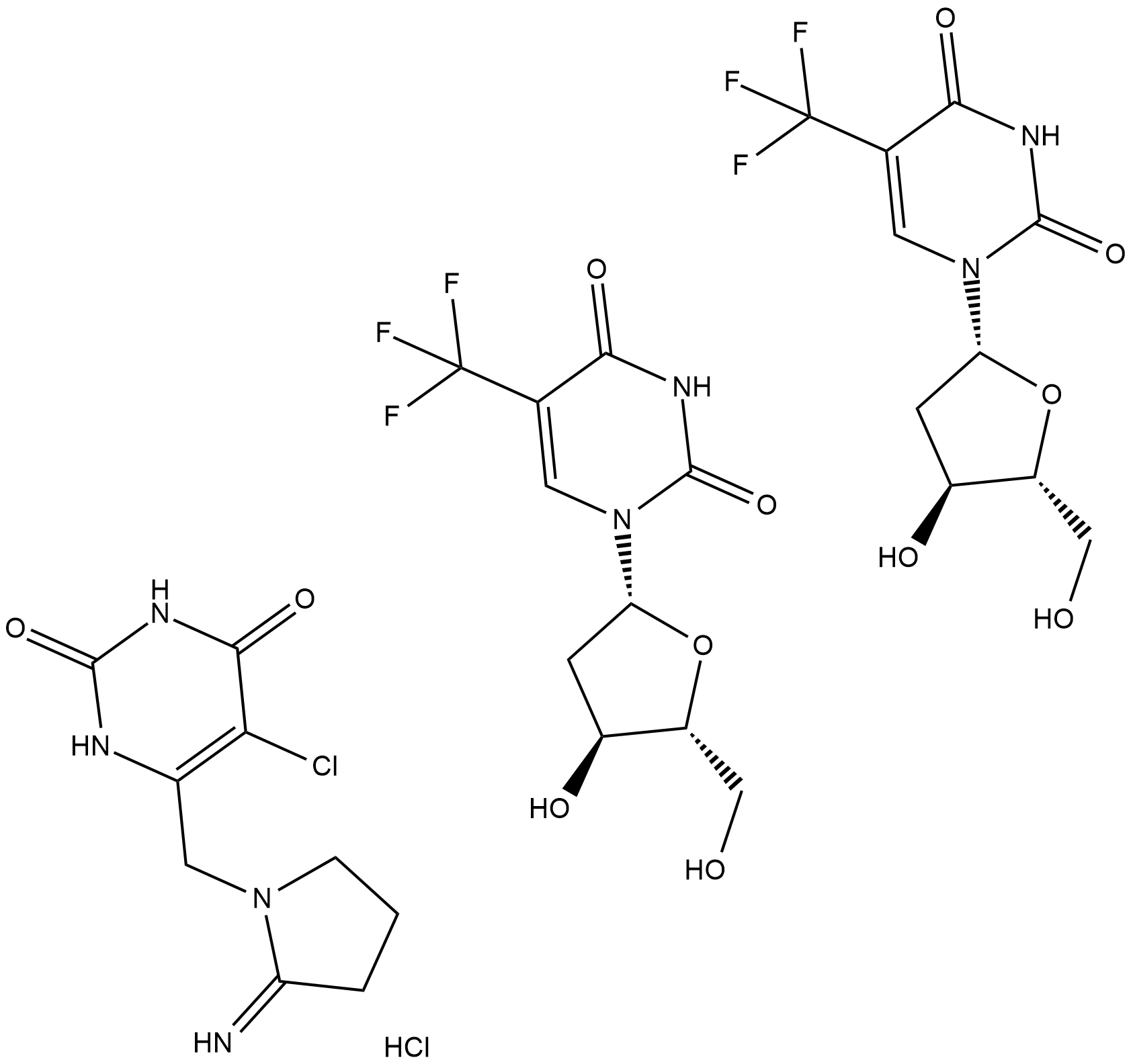
TAS-102 (TAS-102) es un agente antitumoral nucleÓsido potente y activo por vÍa oral. La composiciÓn de TAS-102 (TAS-102) es una mezcla 1:0,5 (en base molar) de alfa,alfa,alfa-trifluorotimidina (FTD) e inhibidor de timidina fosforilasa (TPI). TAS-102 (TAS-102) muestra la actividad antitumoral principalmente a través de la inhibiciÓn de la timidilato sintasa (TS) y la incorporaciÓn al ADN.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 733030-01-8
Sample solution is provided at 25 µL, 10mM.
TAS-102 is a novel oral combination drug that consists of an antineoplastic thymidine-based nucleoside analog, trifluorothymidine, and a potent thymidine phosphorylase inhibitor, tipiracil, in a 1:0.5 molar ratio.
TAS-102, a novel antimetabolite combination chemotherapy agent, consists of a rediscovered antimetabolite agent, trifluorothymidine (trifluridine, FTD) combined with the metabolic inhibitor of thymidine phosphorylase, tipiracil (TPI), in a 1:0.5 molar ratio[1]. FTD is the active antitumor component of TAS-102; its monophosphate form inhibits thymidylate synthase, and its triphosphate form is incorporated into DNA in tumor cells. The incorporation into DNA is known to have antitumor effects, since the inhibition of thymidylate synthase caused by oral FTD rapidly disappears after the drug's elimination. When FTD is administered orally, it is rapidly degraded to its inactive form by thymidine phosphorylase in the intestines and liver (first-pass effect). Consequently, TPI is synthesized to maintain adequate plasma concentrations of orally-administered FTD and to potentiate the antitumor activity of FTD[2].
TAS-102 and CPT-11 is a promising treatment option for colorectal or gastric cancer. TAS-102 monotherapy has a significant antitumor activity against KM12C/5-FUFU-bearing nude mice. The combination-treated (CPT-11-and TAS-102) group is significantly superior to monotherapy[2]. FTD systemic exposure in plasma increaseS dose-dependently. The tumor growth rate and body weight gain decreaseS dose-dependently, but FTD concentrations in the DNA of tumor tissues and white blood cells increases dose-dependently. FTD inhibits colony formation of bone marrow cells in a concentration-dependent manner[3].
References:
[1]. Uboha N, et al. TAS-102: a novel antimetabolite for the 21st century. Future Oncol. 2016 Jan;12(2):153-63.
[2]. Nukatsuka M, et al. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, with irinotecan hydrochloride on human colorectal and gastric cancer xenografts. Anticancer Res. 2015 Mar;35(3):1437-45.
[3]. Yamashita F, et al. Exposure-dependent incorporation of trifluridine into DNA of tumors and white blood cells in tumor-bearing mouse. Cancer Chemother Pharmacol. 2015 Aug;76(2):325-33.
Average Rating: 5 (Based on Reviews and 16 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















