Thailanstatin A |
| Catalog No.GC62288 |
La thailanstatina A es un inhibidor ultrapotente del empalme del ARN eucariÓtico (IC50=650 nM). La thailanstatina A ejerce efectos a través de la uniÓn no covalente a la subunidad SF3b del subcomplejo U2 snRNA del spliceosoma y muestra IC50 de nM bajo a sub-nM contra mÚltiples lÍneas celulares de cÁncer. La thailanstatina A, una carga Útil para los ADC, se conjuga con las lisinas en trastuzumab y produce ADC \Thailanstatin A, a payload for ADCs, is conjugated to the lysines on trastuzumab yielding "linker-less" ADC.en_es_2021q4.md
Products are for research use only. Not for human use. We do not sell to patients.
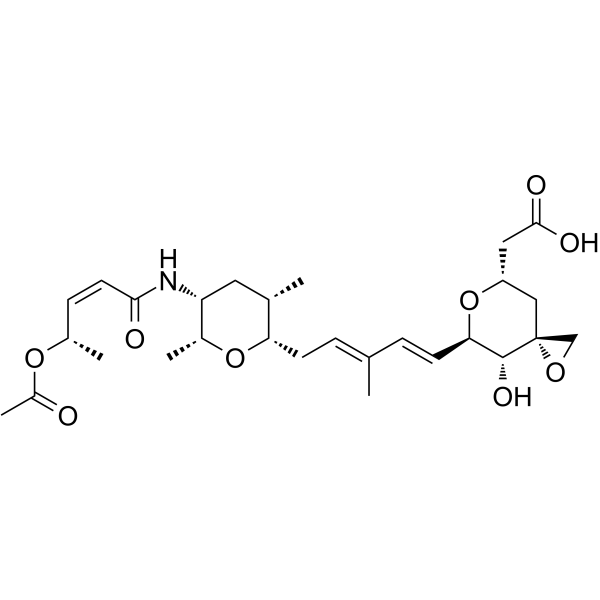
Cas No.: 1426953-21-0
Sample solution is provided at 25 µL, 10mM.
Thailanstatin A is an ultra-potent inhibitor of eukaryotic RNA splicing (IC50=650 nM). Thailanstatin A exerts effects via non-covalent binding to the SF3b subunit of the U2 snRNA subcomplex of the spliceosome and shows low-nM to sub-nM IC50s against multiple cancer cell lines. Thailanstatin A, a payload for ADCs, is conjugated to the lysines on trastuzumab yielding "linker-less" ADC[1][2][3].
Thailanstatin A (TST-A) is a potent antiproliferative natural product discovered by our group from Burkholderia thailandensis MSMB43[2].Thailanstatin A (DU-145, NCI-H232A, MDA-MB-231 and SKOV-3 cells) exhibits potent antiproliferative activities with GI50s in the single nM range (1.11-2.69 nM)[3].
[1]. Puthenveetil S, et al. Natural Product Splicing Inhibitors: A New Class of Antibody-Drug Conjugate (ADC) Payloads. Bioconjug Chem. 2016;27(8):1880-1888.
[2]. Ghosh AK, et al. Enantioselective Synthesis of Thailanstatin A Methyl Ester and Evaluation of in Vitro Splicing Inhibition. J Org Chem. 2018;83(9):5187-5198.
[3]. Liu X, et al. Genomics-guided discovery of thailanstatins A, B, and C As pre-mRNA splicing inhibitors and antiproliferative agents from Burkholderia thailandensis MSMB43. J Nat Prod. 2013;76(4):685-693.
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















