Zonisamide (Synonyms: CI-912, PD 110843) |
| Catalog No.GC11735 |
La zonisamida (AD 810) es un inhibidor de la anhidrasa carbÓnica activo por vÍa oral, con Kis de 35,2 y 20,6 nM para hCA II y hCA V, respectivamente.
Products are for research use only. Not for human use. We do not sell to patients.
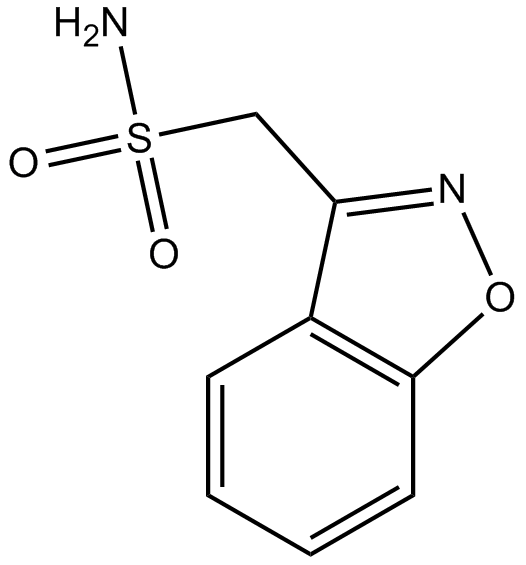
Cas No.: 68291-97-4
Sample solution is provided at 25 µL, 10mM.
Zonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug.Target: Calcium channel inhibitor; Sodium channel inhibitorZonisamide is a sulfonamide anticonvulsant approved for use as an adjunctive therapy in adults with partial-onset seizures for adults; infantile spasm, mixed seizure types of Lennox-Gastaut syndrome, myoclonic, and generalized tonic clonic seizure. Zonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug. It has shown activity in various animal models of epilepsy, and although a detailed mode of action awaits clarification it appears to block the propagation/spread of seizure discharges and to suppress the epileptogenic focus [1].Zonisamide 500 mg/day was significantly superior to placebo in reducing the frequency of complex partial seizures (-51% versus -16%), all partial seizures and all seizures, with dose-dependent benefit provided over a 100-500 mg/day dose range. Supporting trials have confirmed significant increases in reduction in median seizure frequency (up to 41%) and responder rates (35-42%) compared with placebo following zonisamide 400-600 mg/day, enabling 20-27% of patients to attain >or=75% reduction in seizure frequency [2].Clinical indications: Epilepsy; Lewy body dementia; Parkinsons diseaseToxicity: Anorexia; Somnolence; Dizziness; Irritability; Confusional state; Depression; Diplopia; Memory impairment
References:
[1]. Peters DH, et al. Zonisamide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in epilepsy. Drugs. 1993 May;45(5):760-87.
[2]. Brodie MJ, et al. Zonisamide as adjunctive therapy for refractory partial seizures. Epilepsy Res. 2006 Feb;68 Suppl 2:S11-6. Epub 2005 Nov 28.
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















