AG-221 (Enasidenib) (Synonyms: AG-221, CC-90007) |
| رقم الكتالوجGC13147 |
AG-221 (Enasidenib) هو مثبط انتقائي شفهي وقوي وقابل للعكس للإنزيمات المتحولة IDH2 ، مع IC50s من 100 و 400 نانومتر مقابل IDH2R140Q و IDH2R172K ، على التوالي.
Products are for research use only. Not for human use. We do not sell to patients.
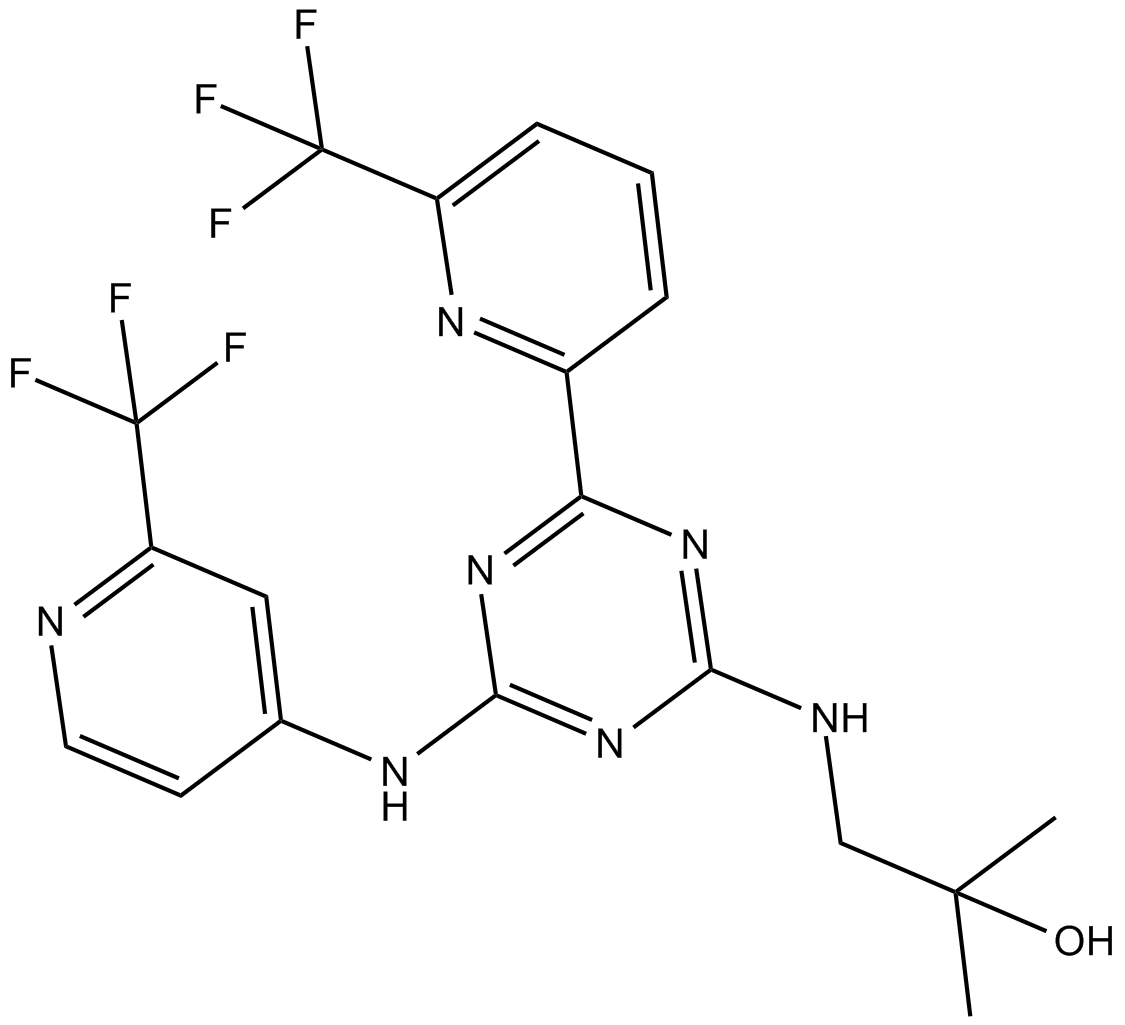
Cas No.: 1446502-11-9
Sample solution is provided at 25 µL, 10mM.
IC50: ~16 nM for IDH2 R140Q mutant
AG-221 (Enasidenib) is a mutant isocitrate dehydrogenase 2 (IDH2) inhibitor.
The somatic mutations of IDH1 and IDH2 are found in patients with acute myeloid leukemia. Leukemia-associated IDH1/2 mutations lead to aberrant accumulation of the oncometabolite 2-hydroxyglutarate (2-HG).
In vitro: AG-221 was found to be able to reduce 2-HG levels by >90%, reverse in-vitro histone and DNA hypermethylation, and induce differentiation in leukemia cell model as well. In addition, a dose dependent proliferative burst of the human specific CD45+ blast cells was observed by the treatment of AG-221, as measured by the expression of CD11b, CD14, CD15 and cell morphology [1].
In vivo: The efficacy of AG-221 in a primary human AML xenograft model with the IDH2 R140Q mutation was studied, and the results showed that AG-221 could reduce 2-HG in the plasma, bone marrow, and urine of engrafted mice potently. In addition, the treatment of AG-221 could also induce a significant and dose dependent survival benefit as demonstrated by that all mice in the high dose treatment of AG-221 survived to the end of study [1].
Clinical trial: A phase 1, multicenter, dose-escalation, safety, PK, PD, and clinical activity study of AG-221 in patients with advanced hematologic malignancies with an IDH2 mutation has been conducted [2].
References:
[1] Kate Ellwood-Yen, Fang Wang, Jeremy Travins, Yue Chen, Hua Yang, Kim Straley, Sung Choe, Marion Dorsch, Sam Agresta, David Schenkein, Scott Biller, Michael Su. AG-221 offers a survival advantage in a primary human IDH2 mutant AML xenograft model. [abstract]. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5-9; San Diego, CA. Philadelphia (PA): AACR; Cancer Res 2014;74(19 Suppl):Abstract nr 3116. doi:10.1158/1538-7445.AM2014-3116
[2] https://clinicaltrials. gov/ct2/show/NCT02577406term=Enasidenib&rank=1
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















