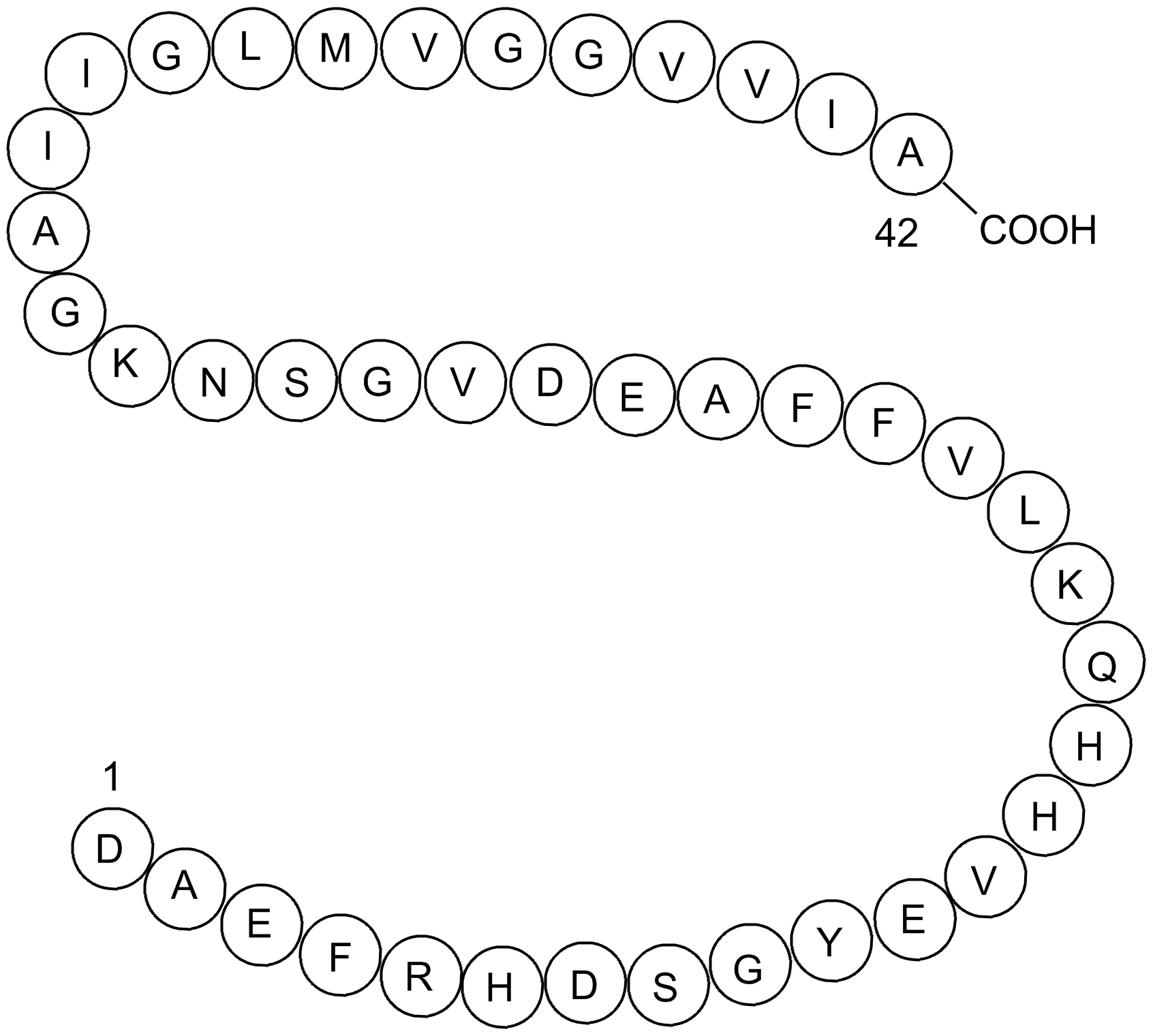
β-Amyloid (1-42), human TFA |
| Catalog No.GC16243 |
β-Amyloid (1-42), human TFA (Amyloid β-Peptide (1-42) (human) TFA) est un peptide de 42 acides aminés qui joue un rÔle clé dans la pathogenèse de la maladie d'Alzheimer.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 107761-42-2
Sample solution is provided at 25 µL, 10mM.
Amyloid β-Peptide (1-42) human TFA is a 42-amino acid peptide. Alzheimer's disease (AD) is characterized phenotypically by memory impairment, neurochemically by accumulation of β-amyloid peptide (such as β-Amyloid (1-42)) and morphologically by an initial loss of nerve terminals in cortical and hippocampal regions [1]. the abnormal production of soluble forms of β-amyloid peptides (Aβ), such as β-Amyloid (1-42), have been proposed as a major culprit in AD [2].
β-Amyloid (1-42) can impair synaptic function, typified by its ability to affect synaptic plasticity [3], and trigger events leading to a loss of viability of synapses [4], and leads to memory impairment [5]. The intracerebroventricular administration of β-Amyloid (1-42) has been proposed as a model of AD [6].
β-Amyloid (1-42) (100 µg/ml) for 24 h cell viability ofSH-SY5Y cells dropped to about 50%, β-Amyloid (1-42)-induced cell apoptosis could be completely prevented by EGb761 at 100 µg/ml and to a lesser extent, by quercetin (1.5 µg/ml) and ginkgolide B (10 µg/ml) [7].
β-Amyloid (1-42) (icv. 2 nmol in 4 µl) caused a predominant loss of glutamatergic and cholinergic markers [1].β-Amyloid (1-42) was combined with inducers of oxidative stress to induce neuronal cell death, amyloid deposits, gliosis and memory impairment following a 4 week intracerebroventricular infusion. Oxidative stress was induced using the pro-oxidative cation Fe2+ and the glutathione synthesis inhibitor buthionine sulfoximine (BSO) [8].
References:
[1].Canas P M, Simões A P, Rodrigues R J, et al. Predominant loss of glutamatergic terminal markers in a β-amyloid peptide model of Alzheimer's disease[J]. Neuropharmacology, 2014, 76: 51-56.
[2].Hardy J, Selkoe D J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics[J]. science, 2002, 297(5580): 353-356.
[3].Venkitaramani D V, Chin J, Netzer W J, et al. β-amyloid modulation of synaptic transmission and plasticity[J]. Journal of Neuroscience, 2007, 27(44): 11832-11837.
[4].Mattson M P, Partin J, Begley J G. Amyloid β-peptide induces apoptosis-related events in synapses and dendrites[J]. Brain research, 1998, 807(1-2): 167-176.
[5].Selkoe D J. Soluble oligomers of the amyloid β-protein: Impair synaptic plasticity and behavior[J]. Synaptic Plasticity and the Mechanism of Alzheimer's Disease, 2008: 89-102.
[6].Lawlor P A, Young D. Aβ infusion and related models of Alzheimer dementia[M]//Animal models of Dementia. Humana Press, 2011: 347-370.
[7].Shi C, Zhao L, Zhu B, et al. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against β-amyloid peptide-induced toxicity in SH-SY5Y cells[J]. Chemico-biological interactions, 2009, 181(1): 115-123.
[8].Lecanu, L., Greeson, J., & Papadopoulos, V. (2006). Beta-amyloid and oxidative stress jointly induce neuronal death, amyloid deposits, gliosis, and memory impairment in the rat brain. Pharmacology, 76(1), 19-33.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















