Anti-hypertensive sulfonanilide 1 |
| Catalog No.GC33770 |
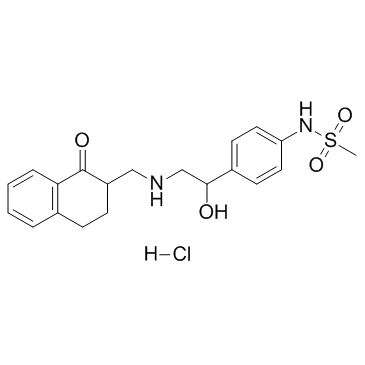
Le sulfonanilide antihypertenseur 1 est un puissant antihypertenseur extrait du brevet EP0338793A2, composé XVIIIa,b*, exemple n°1.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 129280-22-4
Sample solution is provided at 25 µL, 10mM.
Anti-hypertensive sulfonanilide 1 is a potent antihypertensive agent extracted from patent EP0338793A2, compound XVIIIa,b*, example No.1.
Anti-hypertensive sulfonanilide 1 can be classified as tetralin sulfonanilides, is found to have antihypertensive properties and is therefore useful in controlling elevated blood pressure in mammals such as humans. The effective antihypertensive amount for use will vary both with the route of administration, the condition under treatment and the mammal undergoing treatment, and is ultimately at the discretion of the physician. A suitable oral dose of the active compound for a mammal is in the range of from about 1 to about 50 mg per kilogram body weight per day; preferably from about 2 to about 20 mg/kg[1].
[1]. Mcdermed J, et al. Anti-hypertensive sulfonanilides. EP0338793A2.
Average Rating: 5 (Based on Reviews and 2 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















