BMF-219 |
| Catalog No.GC25159 |
BMF-219 est un nouvel inhibiteur de la menine puissant et irréversible, qui peut être utilisé dans le traitement de la leucémie.
Products are for research use only. Not for human use. We do not sell to patients.
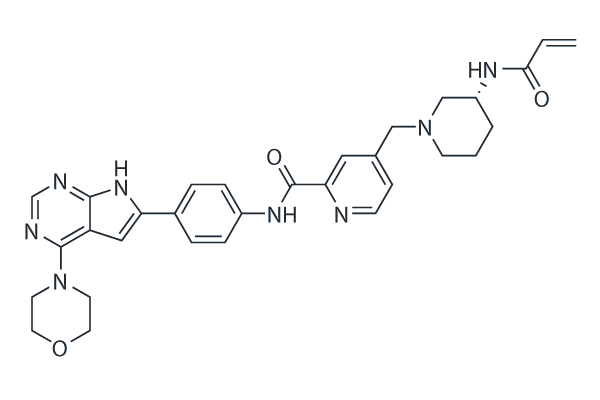
Cas No.: 2448172-22-1
Sample solution is provided at 25 µL, 10mM.
BMF-219 is a highly selective and irreversible inhibitor of menin which has shown very promising activity in in-vitro and in-vivo preclinical tumour models[1-3].
BMF-219(6 or 24 hours; 0.5 or 1 µM) treatment significantly reduced MYC(v-myc avian myelocytomatosis viral oncogene homolog) transcription levels in cells[4].
BMF-219(40-200mg/kg; 28days; p.o) significantly reduces HbA1c and controls blood glucose levels in a 4-week dosing study in ZDF rats. BMF-219 significantly reduces blood lipemic levels and body weight in Streptozotocin (STZ) rats[5-6]. A comprehensive panel of Chronic Lymphocytic Leukemia (CLL) samples isolated from patients with Rai Stage 1 to 3 disease were cultured ex vivo in the presence of BMF-219 to assess the antileukemic activity. BMF-219 demonstrated high potency, achieving > 98% cell lethality at 1 µM exposure with IC50 values in the range of 0.1 to 0.38 µM[7].
References:
[1]. Dempke WCM, Desole M, et. al. Targeting the undruggable: menin inhibitors ante portas. J Cancer Res Clin Oncol. 2023 Sep;149(11):9451-9459. doi: 10.1007/s00432-023-04752-9. Epub 2023 Apr 27. PMID: 37103568.
[2]. Farhad Ravandi et al., COVALENT-101: A phase 1 study of BMF-219, a novel oral irreversible menin inhibitor, in patients with relapsed/refractory (R/R) acute leukemia (AL), diffuse large B-cell lymphoma (DLBCL), and multiple myeloma (MM). JCO 40, TPS7064-TPS7064(2022). DOI:10.1200/JCO.2022.40.16_suppl.TPS7064
[3]. JOSE E. RODRIGUEZ, et. al. 91-LB: COVALENT-111, a Phase 1/2 Trial of BMF-219, a Covalent Menin Inhibitor, in Patients with Type 2 Diabetes Mellitus—Preliminary Results. Diabetes 20 June 2023; 72 (Supplement_1): 91-LB. https://doi.org/10.2337/db23-91-LB
[4]. Priyanka Somanath, Daniel Lu,et. al. Novel Irreversible Menin Inhibitor, BMF-219, Shows Potent Single Agent Activity in Clinically Relevant DLBCL Cells. DOI: 10.1182/blood-2021-148045
[5]. PRIYANKA SOMANATH, et. al. 113-LB: Oral Menin Inhibitor, BMF-219, Displays a Significant and Durable Reduction in HbA1c in a Type 2 Diabetes Mellitus Rat Model. Diabetes 1 June 2022; 71 (Supplement_1): 113-LB. https://doi.org/10.2337/db22-113-LB
[6]. THOMAS BUTLER, et. al. 851-P: Oral Long-Acting Menin Inhibitor Normalizes Type 2 Diabetes Mellitus (T2DM) in Two Rat Models. Diabetes 1 June 2022; 71 (Supplement_1): 851-P. https://doi.org/10.2337/db22-851-P
[7]. Priyanka Somanath et al., Preclinical activity of irreversible Menin inhibitor, BMF-219, in chronic lymphocytic leukemia. JCO 40, 7541-7541(2022). DOI:10.1200/JCO.2022.40.16_suppl.7541
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















