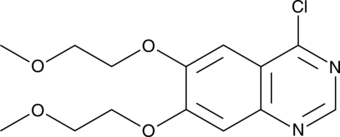
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline |
| Catalog No.GC46628 |
A building block and synthetic intermediate
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 183322-18-1
Sample solution is provided at 25 µL, 10mM.
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline is a building block and synthetic intermediate.1,2,3,4,5 It has been used as a precursor in the synthesis of receptor tyrosine kinase (RTK) inhibitors, dual RTK and histone deacetylase (HDAC) inhibitors, and anticancer compounds.1,2,3 It is also a synthetic intermediate in the synthesis of EGFR inhibitors, including erlotinib , with antiproliferative activity.4,5
1.Pandey, A., Volkots, D.L., Seroogy, J.M., et al.Identification of orally active, potent, and selective 4-piperazinylquinazolines as antagonists of the platelet-derived growth factor receptor tyrosine kinase familyJ. Med. Chem.45(17)3772-3793(2002) 2.Li, W.W., Wang, X.Y., Zheng, R.L., et al.Discovery of the novel potent and selective FLT3 inhibitor 1-{5-[7-(3-morpholinopropoxy)quinazolin-4-ylthio]-[1,3,4]thiadiazol-2-yl}-3-p-tolylurea and its anti-acute myeloid leukemia (AML) activities in vitro and in vivoJ. Med. Chem.55(8)3852-3866(2012) 3.Zhang, X., Su, M., Chen, Y., et al.The design and synthesis of a new class of RTK/HDAC dual-targeted inhibitorsMolecules18(6)6491-6503(2013) 4.Liu, Z., Wang, L., Feng, M., et al.New acrylamide-substituted quinazoline derivatives with enhanced potency for the treatment of EGFR T790M-mutant non-small-cell lung cancersBioorg. Chem.77593-599(2018) 5.Knesl, P., RÖseling, D., and Jordis, U.Improved synthesis of substituted 6,7-dihydroxy-4-quinazolineamines: Tandutinib, erlotinib and gefitinibMolecules11(4)286-297(2006)
Average Rating: 5 (Based on Reviews and 23 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















