Esonarimod (KE-298) (Synonyms: KE-298) |
| Catalog No.GC31933 |
Esonarimod (KE-298) (KE-298) est un agent antirhumatismal.
Products are for research use only. Not for human use. We do not sell to patients.
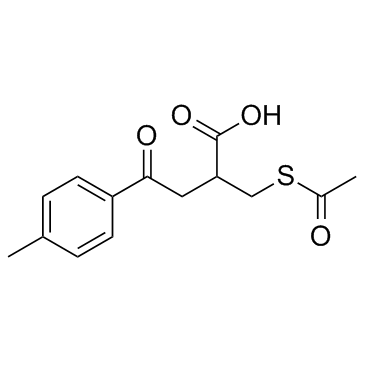
Cas No.: 101973-77-7
Sample solution is provided at 25 µL, 10mM.
Esonarimod is an antirheumatic drug.
Esonarimod (KE-298) (10 to 300 μg/mL) suppresses the production of NO by RAW264.7 cells in a dose dependent manner. The IC50 of Esonarimod is 117.5 μg/mL. Esonarimod does not affect cellular viability at these tested doses. Esonarimod has no direct effect on NOS activity in cell-free extracts of RAW264.7 cells[1].
After repeated oral administration of Esonarimod (14C-KE-298), the radioactivity decreases rapidly and no tendency towards accumulation is found[2].
[1]. Inoue T, et al. KE-298 and its active metabolite KE-758 suppress nitric oxide production by murine macrophage cells and peritoneal cells from rats with adjuvant induced arthritis. J Rheumatol. 2001 Jun;28(6):1229-37. [2]. Hasegawa M, et al. Formation of a disulfide protein conjugate of the SH-group-containing metabolite (M-I) ofesonarimod (KE-298) and its elimination in rats. J Pharm Pharmacol. 2002 Apr;54(4):493-8.
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















