L-Canaline |
| Catalog No.GC14954 |
La L-Canaline est un acide aminé non protéique stocké dans de nombreuses plantes légumineuses.
Products are for research use only. Not for human use. We do not sell to patients.
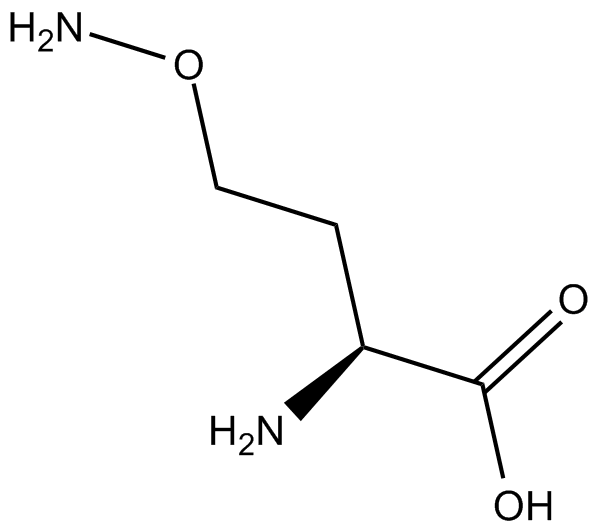
Cas No.: 496-93-5
Sample solution is provided at 25 µL, 10mM.
L-Canaline is a well-known irreversible inhibitor of ornithine aminotransferase (OAT). The natural L-enantiomer reacts by oxime formation with pyridoxal 5′-phosphate in the active site of the enzyme [1]. L-Canaline is naturally found in plants such as legumes, and has been involved in the metabolism of L-canavanine, an aminooxy analog of arginine [2].
Ornithine aminotransferase (OAT) is a mitochondrial enzyme involved in catalyzing the interaction of L-ornithine and α-ketoglutarate to produce glutamic-y-semialdehyde and glutamate [3].
In vitro: Canaline strongly inhibited the activity of pyridoxal-dependent enzymes, including amino acid decarboxylases, 5-hydroxytryptophan decarboxylase, aminotransferases, tyrosine aminotransferase, ornithine transcarbamylase and plasma diamino-oxidase. The canaline inhibition was due to complex formation between canaline and the pyridoxal coenzyme. l-canaline is one of the most potent inhibitors of pyridoxal enzymes. The IC50 value of l-canaline against Ornithine aminotransferase was 3 ×10-6M [4].
In vivo: Intraperitoneal administration of 500 mg of DL-canaline/kg body wt. only produced a transient inhibition of OAT in brain and liver by 65-70%, suggesting that DL-canaline was not a useful tool in studies of biological consequences of OAT inhibition. [1].
References:
[1] Bolkenius F N, Kndgen B, Seiler N. DL-canaline and 5-fluoromethylornithine. Comparison of two inactivators of ornithine aminotransferase[J]. Biochemical Journal, 1990, 268(2): 409-414.
[2] Rosenthal G A, Rhodes D. L-Canavanine transport and utilization in developing jack bean, Canavalia ensiformis (L.) DC.[Leguminosae][J]. Plant physiology, 1984, 76(2): 541-544.
[3] Peraino C, Bunville L G, Tahmisian T N. Chemical, physical, and morphological properties of ornithine aminotransferase from rat liver[J]. Journal of Biological Chemistry, 1969, 244(9): 2241-2249.
[4] Rahiala E L, Kekomki M, Jnne J, et al. Inhibition of pyridoxal enzymes by L-canaline[J]. Biochimica et Biophysica Acta (BBA)-Enzymology, 1971, 227(2): 337-343.
Average Rating: 5 (Based on Reviews and 26 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















