Pyridoxal isonicotinoyl hydrazone (Synonyms: NSC 77674, PIH) |
| Catalog No.GC13308 |
Le pyridoxal isonicotinoyl hydrazone (PIH) est un agent chélateur du Fe tridentate lipophile qui présente une efficacité élevée de chélation du Fe.
Products are for research use only. Not for human use. We do not sell to patients.
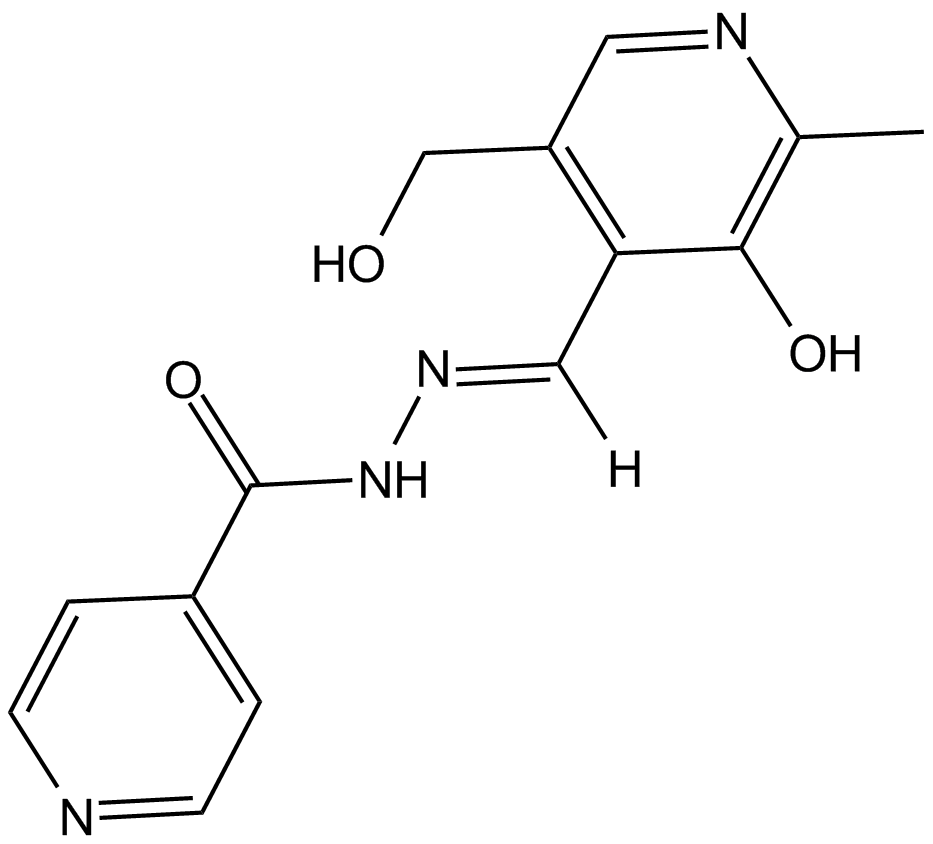
Cas No.: 737-86-0
Sample solution is provided at 25 µL, 10mM.
Pyridoxal isonicotinoyl hydrazine is a cell-permeable and relatively non-toxic iron (Fe3+) chelator [1].
Pyridoxal isonicotinoyl hydrazone (PIH) is a tridentate Fe-chelating agent that shows high Fe chelation efficacy. In 59Fe-labeled reticulocytes, PIH (0.1 mmol/L) released 38.6% of cellular 59Fe [1]. PIH inhibited the formation of ascorbyl radical and Fe(III)–EDTA-mediated ascorbate oxidation in a dose-dependent way [3].
In Fe-loaded rats, PIH orally administrated resulted in an eightfold increase in fecal Fe excretion and possibly some urinary excretion of Fe [1]. In mice loaded with iron-acetohydroxamic acid complex, pyridoxal isonicotinoyl hydrazone (po.) were given daily for four days at 300 mg/kg/day. Total iron excreted over the 4-day period (micrograms/mouse) was 31 [2]. Pyridoxal isonicotinoyl hydrazine could be used for experimental chelating therapy in iron-overload diseases [3].
References:
[1].Richardson DR, Ponka P. Pyridoxal isonicotinoyl hydrazone and its analogs: potential orally effective iron-chelating agents for the treatment of iron overload disease. J Lab Clin Med. 1998 Apr;131(4):306-15.
[2].Gale GR, Litchenberg WH, Smith AB, et al. Comparative iron mobilizing actions of deferoxamine, 1,2-dimethyl-3-hydroxypyrid-4-one, and pyridoxal isonicotinoyl hydrazone in iron hydroxamate-loaded mice. Res Commun Chem Pathol Pharmacol. 1991 Sep;73(3):299-313.
[3].Maurício AQ, Lopes GK, Gomes CS, et al. Pyridoxal isonicotinoyl hydrazone inhibits iron-induced ascorbate oxidation and ascorbyl radical formation. Biochim Biophys Acta. 2003 Mar 17;1620(1-3):15-24.
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















