Valrocemide (Synonyms: N-Valproyl glycinamide, TV1901, VGD) |
| Catalog No.GC17434 |
Le valrocémide (TV1901) est un candidat-médicament antiépileptique prometteur qui présente un large spectre d'activité anticonvulsivante.
Products are for research use only. Not for human use. We do not sell to patients.
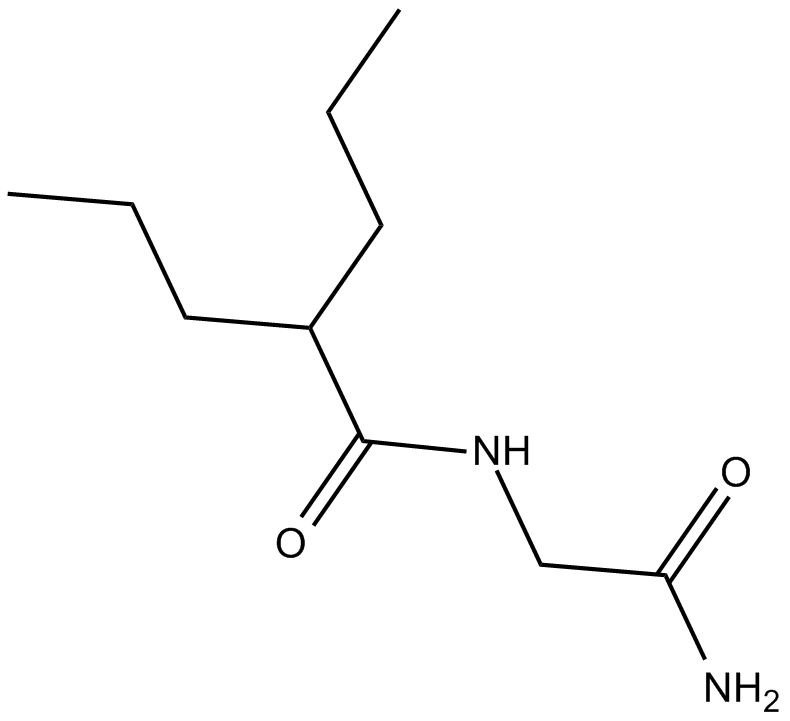
Cas No.: 92262-58-3
Sample solution is provided at 25 µL, 10mM.
Epilepsy is a chronic condition requiring long-term drug treatment, often for the patient's entire life. Valrocemide is an anticonvulsant agent under development by Teva and Acorda as a therapeutic for the treatment of epilepsy.
In vitro: It was found that 1 mM of valrocemide could drastically inhibit human brain crude homogenate MIP synthase activity. Furthermore, the mechanism of the effect of valrocemide were studied and results showed that valrocemide reduced the enzyme activity by an apparent competitive mode of inhibition [1].
In vivo: In mice, valrocemide showed complete protection against maximal electroshock-, pentylenetetrazole-, picrotoxin-, bicuculline-induced seizures as well as 6-Hz "psychomotor" seizures with ED50 values of 151, 132, 275, 248, and 237 mg/kg, respectively. Valrocemide was also effective in preventing sound-induced seizures in Frings audiogenic-seizure susceptible mice. The neurotoxic dose in mice was 332 mg/kg. After oral administration to rats, valrocemide was active in the maximal electroshock test, with an ED50 of 73 mg/kg, and the median neurotoxic dose was 1,000 mg/kg. IP administration of 300 mg/kg of valrocemide to hippocampal kindled SD rats blocked generalized seizures and shortened the afterdischarge duration significantly. Valrocemide also had complete protection from focal seizures in the corneally kindled rats [2].
Clinical trial: Valrocemide is a new antiepileptic drug currently undergoing phase II clinical trials in patients with refractory epilepsy [2].
References:
[1] Shaltiel G,Mark S,Kofman O,Belmaker RH,Agam G. Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol Rep.2007 Jul-Aug;59(4):402-7.
[2] Isoherranen N,Woodhead JH,White HS,Bialer M. Anticonvulsant profile of valrocemide (TV1901): a new antiepileptic drug. Epilepsia.2001 Jul;42(7):831-6.
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















