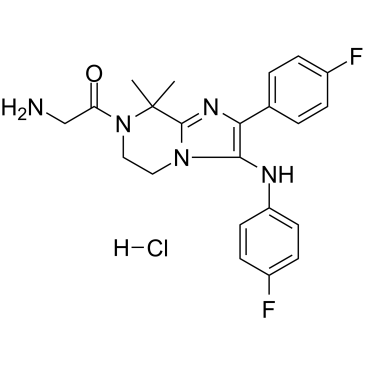
Ganaplacide hydrochloride (Synonyms: KAF156 hydrochloride; GNF156 hydrochloride) |
| Catalog No.GC60864 |
El clorhidrato de Ganaplacide (KAF156) es el primer agente antipalÚdico de imidazolopiperazina oralmente activo de su clase.
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
Ganaplacide (KAF156) hydrochloride is a first-in-class, orally active imidazolopiperazine antimalarial agent. Ganaplacide hydrochloride is active against a broad range of Plasmodium species, including drug-resistant parasites. Ganaplacide hydrochloride is parasiticidal against both asexual and sexual blood stages as well as the liver stages of the parasite[1][2].
Ganaplacide (KAF156) hydrochloride shows blood schizonticidal activity with 50% inhibitory concentrations of 6 to 17.4 nM against P. falciparum drug-sensitive and drug-resistant strains[1].
KAF156 displays cidal activity against mature Plasmodium falciparum gametocytes and thus blocks parasite transmission to Anopheles mosquitoes[1].Ganaplacide (KAF156) hydrochloride (1-15 mg/kg; p.o.) is fully protective in a causal prophylactic mouse model of malaria[1]. Animal Model: Mice (causal prophylactic rodent malaria model)[1]
[1]. Kuhen KL, et al. KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment, and prevention of disease transmission. Antimicrob Agents Chemother. 2014;58(9):5060-5067. [2]. Leong FJ, et al. A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel Imidazolopiperazine KAF156 to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob Agents Chemother. 2014;58(11):6437-6443.
Average Rating: 5 (Based on Reviews and 2 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















