N-Desethyl Sunitinib (Synonyms: SU 12662) |
| Catalog No.GC36710 |
N-desetil sunitinib (SU-12662) es un metabolito de sunitinib. Sunitinib es un potente inhibidor de VEGFR, PDGFRβ y KIT competitivo con ATP con valores de Ki de 2, 9, 17, 8 y 4 nM para VEGFR -1, -2, -3, PDGFRβ y KIT, respectivamente.
Products are for research use only. Not for human use. We do not sell to patients.
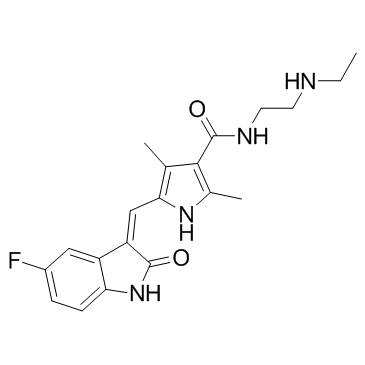
Cas No.: 356068-97-8
Sample solution is provided at 25 µL, 10mM.
N-Desethyl Sunitinib is a metabolite of sunitinib. Sunitinib is a potent, ATP-competitive VEGFR, PDGFRβ and KIT inhibitor with Ki values of 2, 9, 17, 8 and 4 nM for VEGFR -1, -2, -3, PDGFRβ and KIT, respectively.
Sunitinib also potently inhibits Kit and FLT-3[1]. Sunitinib is a potent ATP-competitive inhibitor of VEGFR2 (Flk1) and PDGFRβ with Ki of 9 nM and 8 nM, respectively, displaying >10-fold higher selectivity for VEGFR2 and PDGFR than FGFR-1, EGFR, Cdk2, Met, IGFR-1, Abl, and src. In serum-starved NIH-3T3 cells expressing VEGFR2 or PDGFRβ, Sunitinib inhibits VEGF-dependent VEGFR2 phosphorylation and PDGF-dependent PDGFRβ phosphorylation with IC50 of 10 nM and 10 nM, respectively. Sunitinib inhibits VEGF-induced proliferation of serum-starved HUVECs with IC50 of 40 nM, and inhibits PDGF-induced proliferation of NIH-3T3 cells overexpressing PDGFRβ or PDGFRα with IC50 of 39 nM and 69 nM, respectively[2]. Sunitinib inhibits phosphorylation of wild-type FLT3, FLT3-ITD, and FLT3-Asp835 with IC50 of 250 nM, 50 nM, and 30 nM, respectively. Sunitinib inhibits the proliferation of MV4;11 and OC1-AML5 cells with IC50 of 8 nM and 14 nM, respectively, and induces apoptosis in a dose-dependent manner[3].
Sunitinib (20-80 mg/kg/day) exhibits broad and potent dose-dependent anti-tumor activity against a variety of tumor xenograft models including HT-29, A431, Colo205, H-460, SF763T, C6, A375, or MDA-MB-435, consistent with the substantial and selective inhibition of VEGFR2 or PDGFR phosphorylation and signaling in vivo. Sunitinib (80 mg/kg/day) for 21 days leads to complete tumor regression in six of eight mice, without tumor re-growing during a 110-day observation period after the end of treatment. Second round of treatment with Sunitinib remains efficacious against tumors that are not fully regressed during the first round of treatment. Sunitinib treatment results in significant decrease in tumor MVD, with appr 40% reduction in SF763T glioma tumors. SU11248 treatment results in a complete inhibition of additional tumor growth of luciferase-expressing PC-3M xenografts, despite no reduction in tumor size[2]. Sunitinib treatment (20 mg/kg/day) dramatically suppresses the growth subcutaneous MV4;11 (FLT3-ITD) xenografts and prolongs survival in the FLT3-ITD bone marrow engraftment model[3].
[1]. Sun L, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor r [2]. Mendel DB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Can [3]. O'Farrell AM, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003 May 1;101(9):3597-605. Epub 2003 Jan 16.
Average Rating: 5 (Based on Reviews and 16 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















