Lazertinib (Synonyms: YH25448) |
| Catalog No.GC19218 |
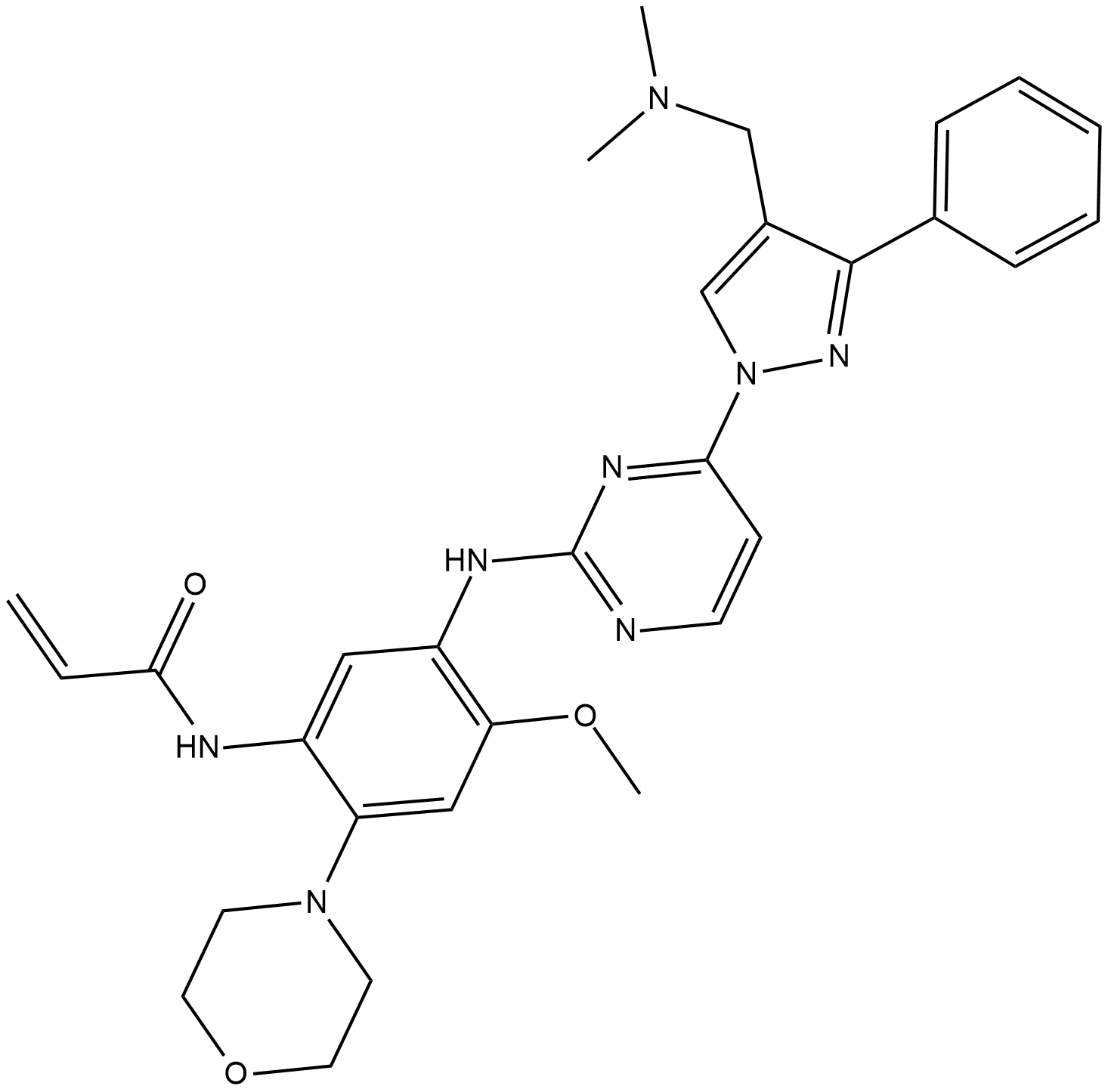
Lazertinib (YH25448) es un potente inhibidor de la tirosina quinasa del EGFR de tercera generaciÓn, altamente selectivo para mutantes, permeable a la barrera hematoencefÁlica, disponible por vÍa oral e irreversible, y puede usarse en la investigaciÓn del cÁncer de pulmÓn de células no pequeÑas.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1903008-80-9
Sample solution is provided at 25 µL, 10mM.
Lazertinib is a potent, highly mutant-selective, blood-brain barrier permeable, orally available and irreversible third-generation EGFR tyrosine kinase inhibitor, and can be used in the research of non-small cell lung cancer.
Lazertinib (YH25448) is a potent, highly mutant-selective, blood-brain barrier permeable, orally available and irreversible third-generation EGFR tyrosine kinase inhibitor, and can be used in the research of non-small cell lung cancer. Lazertinib targets both T790M mutation and activating EGFR mutations (EGFRm) while sparing wild type[1].
References:
[1]. Byoung Chul Cho, et al. YH25448, a 3rd Generation EGFR-TKI, in Patients with EGFR-TKI-resistant NSCLC: Phase I/II Study Results.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















