BMN 673 (Synonyms: Talazoparib) |
| رقم الكتالوجGC15932 |
A PARP inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
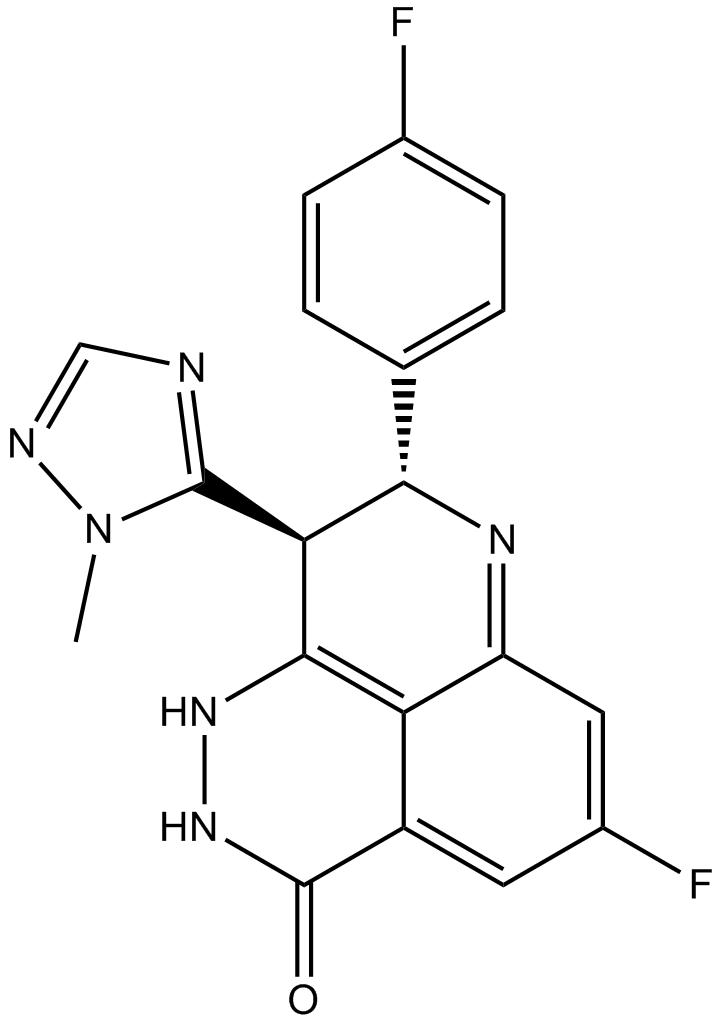
Cas No.: 1207456-01-6
Sample solution is provided at 25 µL, 10mM.
BMN673 is a potent and selective PARP1/2 inhibitor with Ki of 1.2 and 0.9 nM, respectively 1. It had no effect on panels of 72 receptors, ion channels, and enzymes 1. BMN673 showed IC50 value of 0.57 nM in enzymatic assay of PARP1 1. In in vitro assay, it exhibited greater potency than other existing PARP inhibitors, such as veliparib, rucaparib, and olaparib 2. It is also much more potent at trapping PARP-DNA complexes than other PARP inhibitors 3.
BMN673 has shown anti-tumor activity both in vitro and in vivo. It inhibited proliferation of tumor cells and xenografts with defects in homologous recombination 1. The combination of BMN673 and DNA-damaging agents demonstrated synergistic anti-tumor effects 1. In addition, study showed that the expression levels of DNA repair proteins and status of PI3K pathway predict response to BMN673 in small cell lung cancer 4.
BMN673 is currently under investigation in multiple clinical trials for advanced solid tumors or hematological malignancies, either as monotherapy or in combination with other anti-tumor agents.
References:
1. Shen Y, Rehman FL, Feng Y et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res 2013; 19: 5003-5015.
2. Cardnell RJ, Byers LA. Proteomic Markers of DNA Repair and PI3K Pathway Activation Predict Response to the PARP Inhibitor BMN 673 in Small Cell Lung Cancer--Response. Clin Cancer Res 2014; 20: 2237.
3. Murai J, Huang SY, Renaud A et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther 2014; 13: 433-443.
4. Cardnell RJ, Feng Y, Diao L et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res 2013; 19: 6322-6328.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















