Pazopanib Hydrochloride (Synonyms: GW786034;Votrient;Armala;GW 786034;GW-786034) |
| رقم الكتالوجGC12730 |
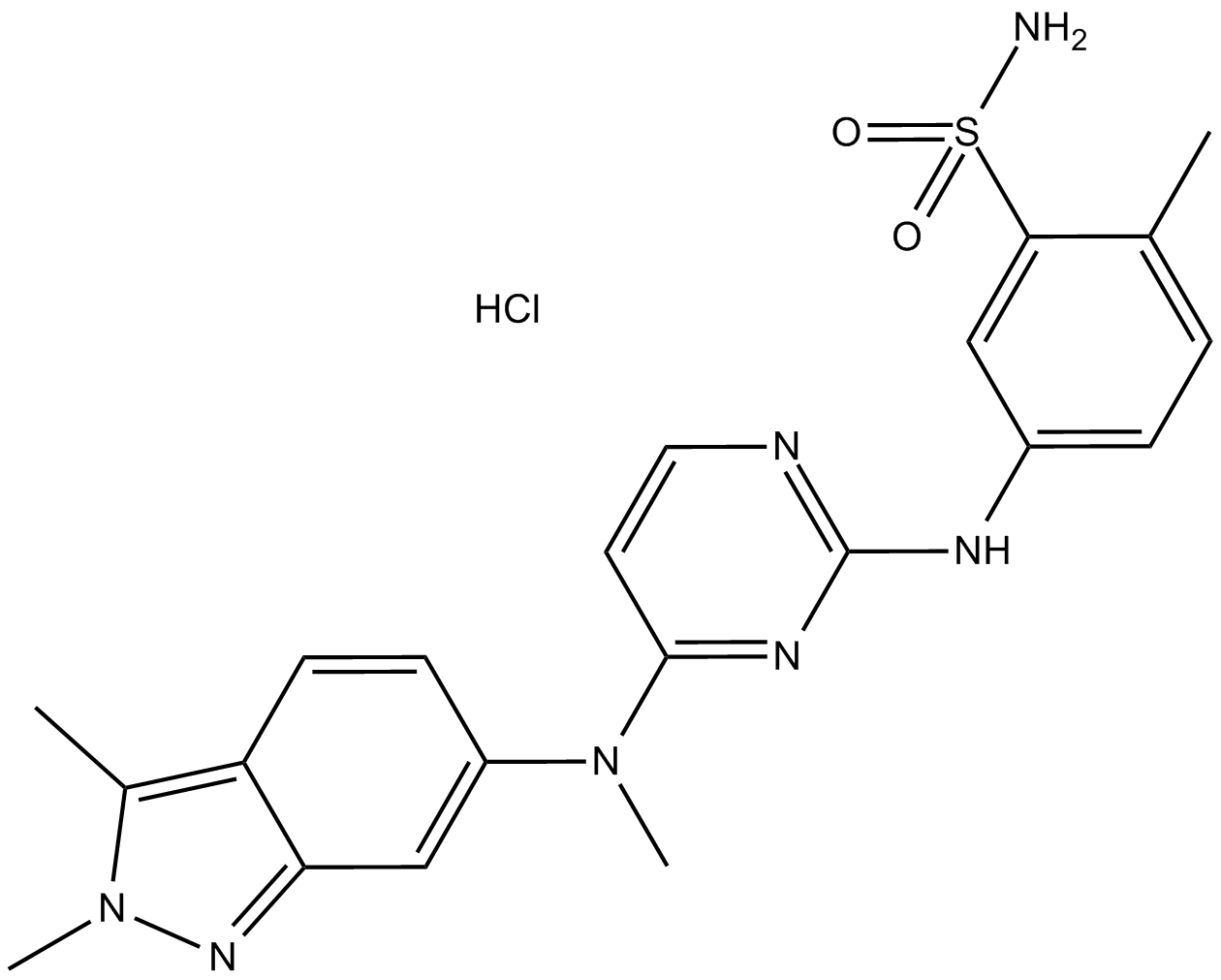
Pazopanib Hydrochloride (GW786034 Hydrochloride) هو مثبط جديد متعدد الأهداف لـ VEGFR1 و VEGFR2 و VEGFR3 و PDGFRβ و c-Kit و FGFR1 و c-Fms مع IC50 من 10 و 30 و 47 و 84 و 74 و 140 و 146 نانومتر ، على التوالى.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 635702-64-6
Sample solution is provided at 25 µL, 10mM.
Pazopanib HCl is a receptor tyrosine kinase inhibitor that targets multiple kinases, including VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit, and c-Fms, with IC50s 10nM, 30nM, 47nM, 84nM, 74nM, 140nM, and 146nM respectively [1-3]. Pazopanib inhibits both tumor growth and angiogenesis through suppressing these targets. In preclinical studies, it has shown anti-tumor activity against several human tumor xenografts, including renal, prostate, colon, lung, melanoma, head and neck, and breast cancer [4]. It also showed desirable pharmacokinetics and oral bioavailability in animal models [4].
Pazopanib has been approved for advanced/metastatic renal cell carcinoma and advanced soft tissue sarcomas by multiple regulatory administrations worldwide, including FDA, EMA, MHRA and TGA. In the clinical trial for soft tissue sarcomas, Pazopanib improved median progression-free survival (PFS) to 4.6 months compared to 1.6 months for patients receiving placebo [5]. In the trial for renal cell carcinoma, Pazopanib increased the median PFS from 4.2 monthe (placebo) to 9.2 months [5]. The most common adverse effect of Pazopanib were diarrhea, hypertension, hair color change, nausea, fatigue, anorexia, and vomiting [6].
References:
1. Verweij J, Sleijfer S. Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert Opin Pharmacother 2013; 14: 929-935.
2. Pick AM, Nystrom KK. Pazopanib for the treatment of metastatic renal cell carcinoma. Clin Ther 2012; 34: 511-520.
3. Bukowski RM, Yasothan U, Kirkpatrick P. Pazopanib. Nat Rev Drug Discov 2010; 9: 17-18.
4. Sonpavde G, Hutson TE. Pazopanib: a novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep 2007; 9: 115-119.
5. http://www.cancer.gov/cancertopics/druginfo/fda-pazopanibhydrochloride
6. http://www.gsksource.com/gskprm/en/US/adirect/gskprm?cmd=ProductDetailPage&product_id=1336067580985&featureKey=603422
Average Rating: 5 (Based on Reviews and 33 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















