A-966492 |
| Catalog No.GC12390 |
A PARP1 and PARP2 inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 934162-61-5
Sample solution is provided at 25 µL, 10mM.
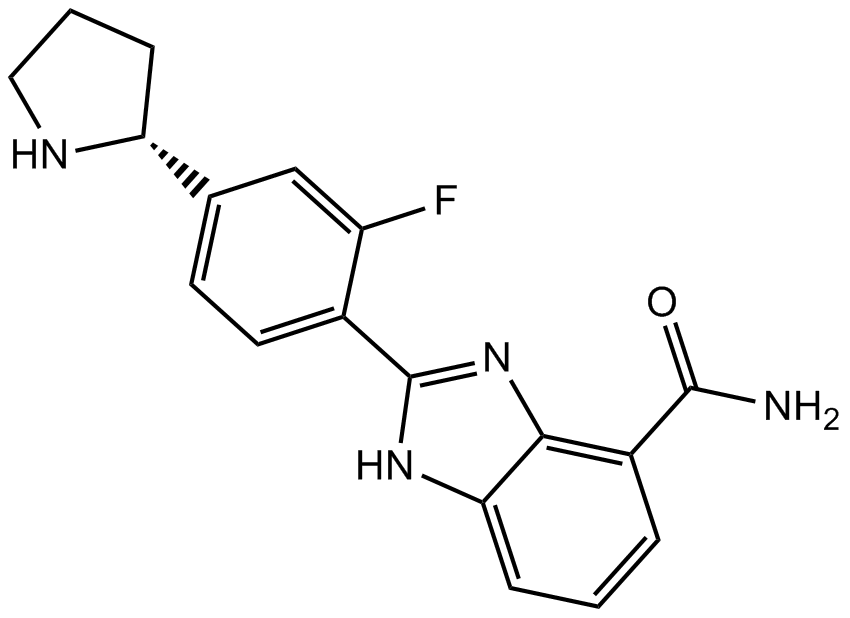
A-966492 is an inhibitor of PARP-1 with Ki value of 1nM [1].
PARP-1 belongs to the poly(ADP-ribose) polymerases (PARPs) family, it contributes to the resistance happened after cancer therapy. A-966492 is a potent inhibitor of both PARP-1 and PARP-2 (Ki value of 1.5nM) with good potency in C41 whole cells (EC50 value of 1nM). A-966492 shows excellent pharmaceutical properties with oral bioavailabilities of 34-72% and half-lives of 1.7-1.9 h. Additionally, A-966492 can crosses the blood-brain barrier. A-966492 is proved potent in a murine B16F10 syngeneic melanoma model and a BRCA1-deficient MX-1 breast carcinoma model. Meanwhile, it can enhance the efficacy of TMZ and carboplatin in these models [1].
References:
[1] Penning TD, Zhu GD, Gong J, Thomas S, Gandhi VB, Liu X, Shi Y, Klinghofer V, Johnson EF, Park CH, Fry EH, Donawho CK, Frost DJ, Buchanan FG, Bukofzer GT, Rodriguez LE, Bontcheva-Diaz V, Bouska JJ, Osterling DJ, Olson AM, Marsh KC, Luo Y, Giranda VL. Optimization of phenyl-substituted benzimidazole carboxamide poly(ADP-ribose) polymerase inhibitors: identification of (S)-2-(2-fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benzimidazole-4-carboxamide (A-966492), a highly potent and efficacious inhibitor. J Med Chem. 2010 Apr 22;53(8):3142-53.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















