Amiselimod hydrochloride (Synonyms: MT-1303) |
| Catalog No.GC19031 |
Le chlorhydrate d'amisélimod est un nouveau modulateur du récepteur 1-phosphate de sphingosine 1 (S1P1), conÇu pour réduire les effets de bradycardie associés au fingolimod et À d'autres modulateurs du récepteur S1P.
Products are for research use only. Not for human use. We do not sell to patients.
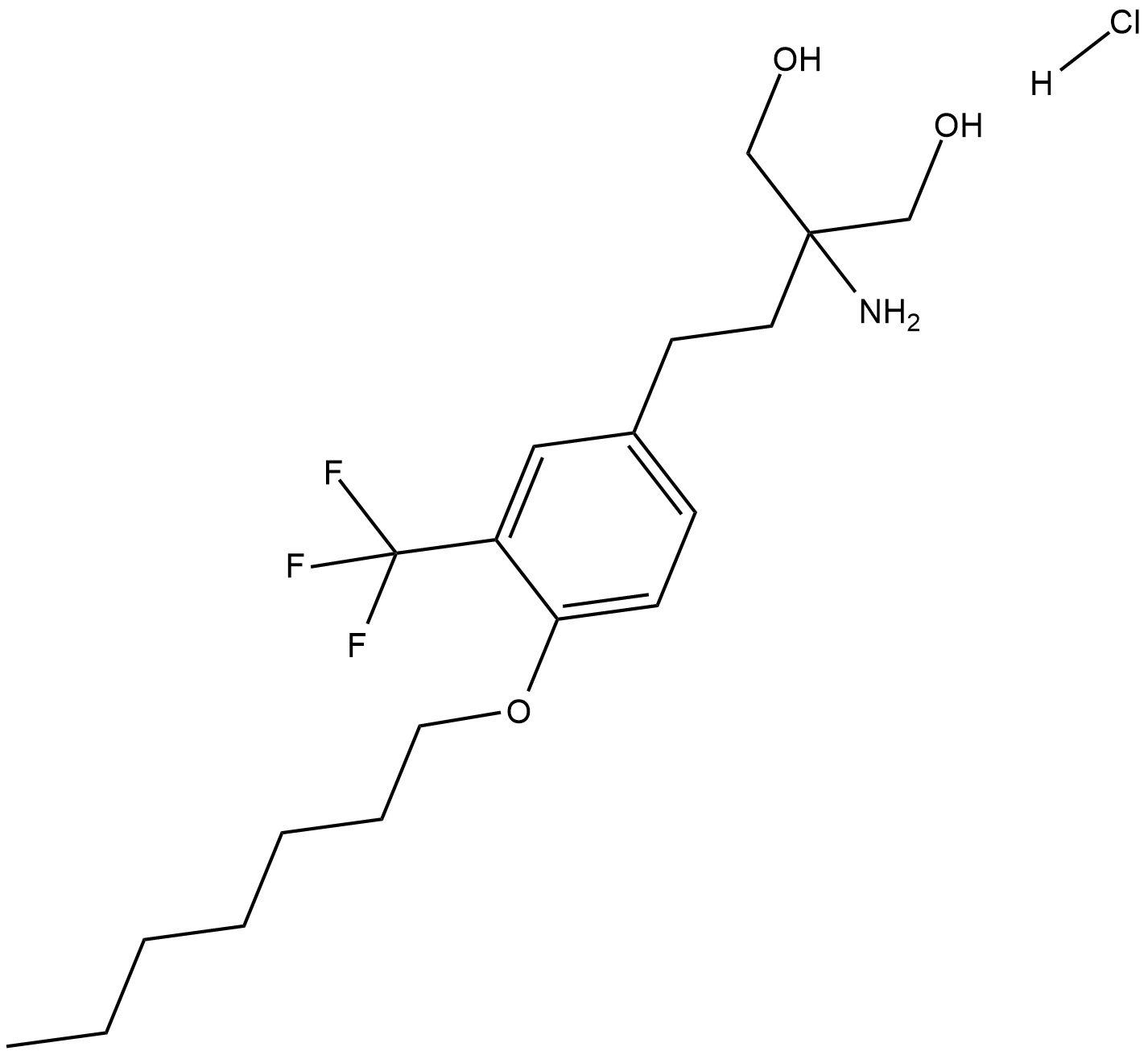
Cas No.: 942398-84-7
Sample solution is provided at 25 µL, 10mM.
Amiselimod hydrochloride is a novel sphingosine 1-phosphate receptor-1 (S1P1) modulator, designed to reduce the bradycardia effects associated with fingolimod and other S1P receptor modulators.target: S1P1In vivo: After oral administration of amiselimod or fingolimod at 1 mg/kg, the concentration of amiselimod-P in rat heart tissue was relatively lower than that of fingolimod-P, potentially contributing to the minimal cardiac effects of amiselimod. Amiselimod-P showed potent selectivity for S1P1 , high selectivity for S1P5 , minimal agonist activity for S1P4 , no distinct agonist activity for S1P2 or S1P3 , and approximately 5-fold weaker GIRK activation than fingolimod-P. [1] Amiselimod 0.2 mg and 0.4 mg significantly reduced the total number of gadolinium-enhanced T1-weighted lesions. [2]
References:
[1]. Sugahara K et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br J Pharmacol. 2016 Oct 7.
[2]. Kappos L et al. Safety and efficacy of amiselimod in relapsing multiple sclerosis (MOMENTUM): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016 Oct;15(11):1148-59.
Average Rating: 5 (Based on Reviews and 8 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















