ASTX660 (Synonyms: Tolinapant) |
| Catalog No.GC32803 |
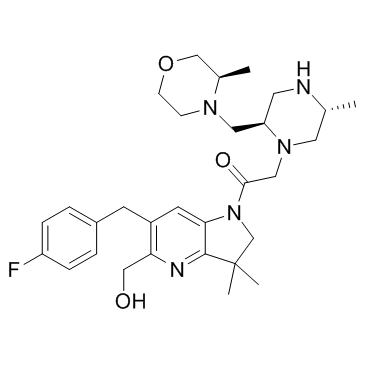
ASTX660 est un double antagoniste biodisponible par voie orale de l'inhibiteur cellulaire de la protéine d'apoptose (cIAP) et de l'inhibiteur lié À l'X de la protéine d'apoptose (XIAP).
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1799328-86-1
Sample solution is provided at 25 µL, 10mM.
ASTX660 is an orally bioavailable dual antagonist of cellular inhibitor of apoptosis protein (cIAP) and X-linked inhibitor of apoptosis protein (XIAP).
ASTX660 is an orally bioavailable dual antagonist of cIAP and XIAP, currently being investigated in a single-agent Phase 1/2 clinical trial in patients with advanced solid tumors and lymphomas. Twenty-one triple-negative breast cancer (TNBC) cell lines are treated with ASTX660 in vitro and it is found that 43% are sensitive to ASTX660[1].
In HCC1806 xenografts in mice, ASTX660 (daily oral treatment) causes moderate tumor growth inhibition but not regression[1].
[1]. Tomoko Smyth, et al. Abstract 1287: The dual IAP antagonist, ASTX660, increases the anti-tumor activity of paclitaxel in preclinical models of triple-negative breast cancer in vivo. Cancer Res 2016;76(14 Suppl).
Average Rating: 5 (Based on Reviews and 38 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















