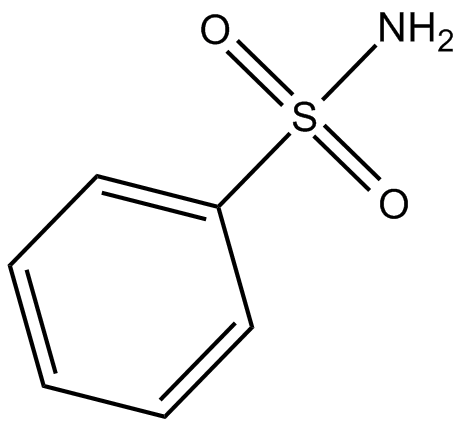
Benzenesulfonamide |
| Catalog No.GC17003 |
carbonic anhydrase inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1998/10/2
Sample solution is provided at 25 µL, 10mM.
Benzenesulfonamide, the amide of benzenesulfonic acid, has been used to produce various derivatives, especially those used as intermediates in the synthesis of photochemicals, dyes, disinfectants, as well as pharmaceuticals.
In vitro: In a previous study, a series of N-aryl-β-alanine- and diazo-derivatives of benzenesulfonamide were designed, synthesized, and their binding affinities to carbonic anhydrases (CA) I, II, VI, VII, XII, and XIII was investigated by the use of isothermal titration calorimetry and fluorescent thermal shift assay. The results indicated that 4-substituted diazobenzenesulfonamides were found to be most potent CA binders among the synthesized derivatives. In addition, the majority of the N-aryl-β-alanine derivatives had better affinity for CA II while diazobenzenesulfonamides showed nanomolar affinities towards CA I isozyme. Moreover, the X-ray crystallographic data showed the binding modes of both derivative groups [1].
In vivo: In the rat CPE model, the most potnet benzenesulfonamide indole derivative at 10 mg/kg in the MC/TW formulation displayed oral efficacy. Moreover, this compound, when administered in another preferred, minimal formulation in the same in vivo model, demonstrated superior oral efficacy to the lead phenylmethane sulfonamide WAY-196025 orally administered in a lipid-based formulation. In addition, this benzenesulfonamide indole derivative was also orally efficacious at 1 mg/kg by attenuating both LAR and the associated AHR to aerosolized carbachol in naturally sensitized sheep, which had been challenged through the airways with A. suum antigen [2].
Clinical trial: Up to now, benzenesulfonamide is still in the preclinical development stage.
References:
[1] Rutkauskas K et al. 4-amino-substituted benzenesulfonamides as inhibitors of human carbonic anhydrases. Molecules. 2014 Oct 28;19(11):17356-80.
[2] Lee KL et al. Benzenesulfonamide indole inhibitors of cytosolic phospholipase A2α: Optimization of in vitro potency and rat pharmacokinetics for oral efficacy. Bioorganic and Medicinal Chemistry. 2008 16(3), 1345-1358.
Average Rating: 5 (Based on Reviews and 35 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















