Daclatasvir (BMS-790052) |
| Catalog No.GC12057 |
HCV NS5A inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
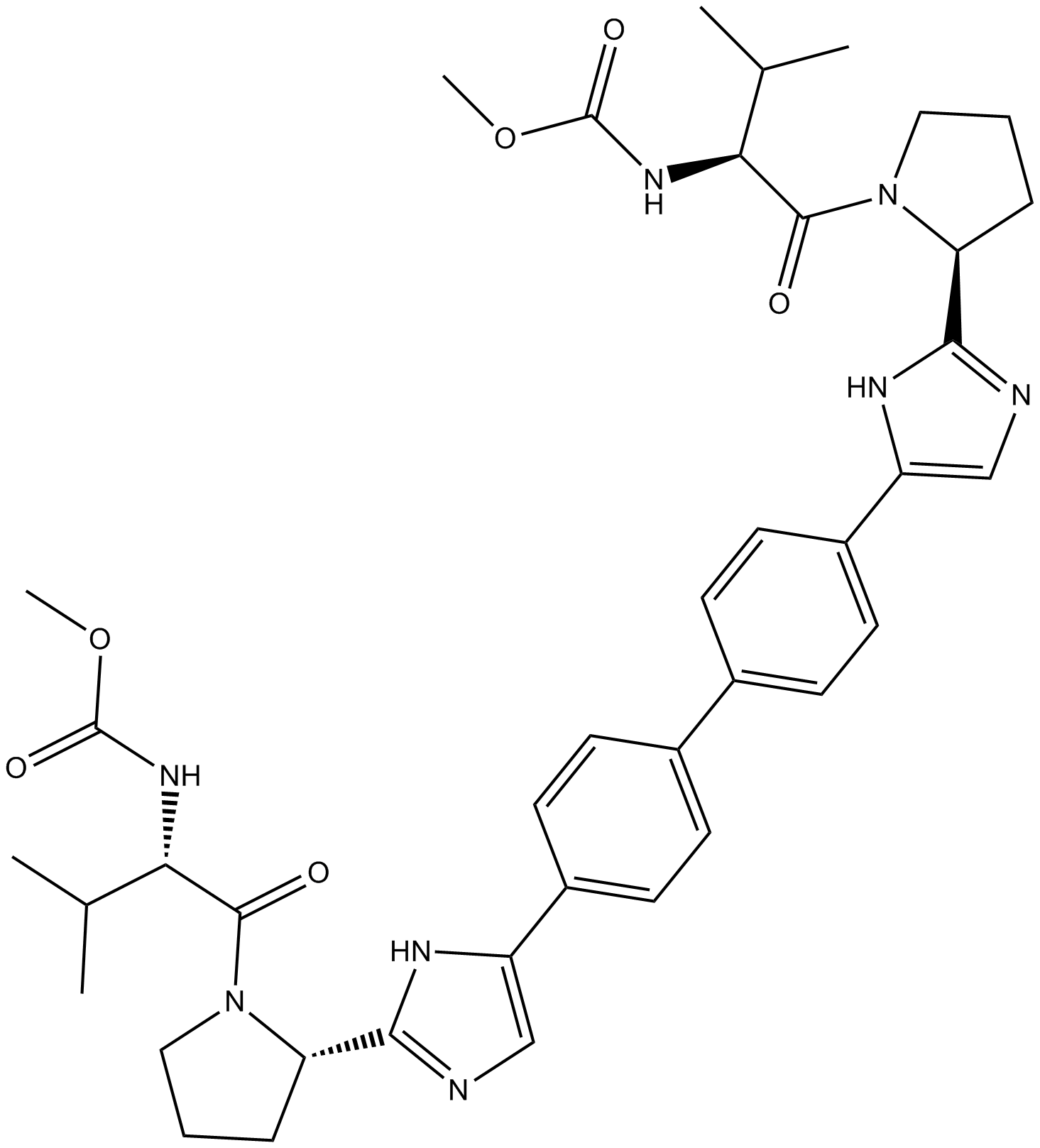
Cas No.: 1214735-16-6
Sample solution is provided at 25 µL, 10mM.
Daclatasvir, formerly known as BMS-790052, is a potent NS5A inhibitor with EC50 values varying from 9 to 146 pM. [1,2]
The nonstructural 5A (NS5A) is a target in HCV drug development, which is a 447 amino-acid, zinc-binding phosphoprotein who plays an essential but enigmatic role in the virus life cycle. However the function of NS5A has no enzymatic activities, which makes it very difficult to understand the antiviral function of daclatasvir. It is assumed that Daclatasvir may interfere with the dimeric structure of NS5A, effecting subtle structural distortions that interfere with protein function in a specific way.[1,2]
The antiviral activity of daclatasvir towards genotypes was assessed by using replication-competent 1a or 1b replicons to construct hybrids in which the entire NS5A coding region or the first 100 amino acids of NS5A from different genotypes replaced the corresponding sequence of the parent replicon. Daclatasvir was reported to be highly potent across all HCV genotypes with half-maximum effective concentrations (EC50) ranging from 9 to 146 pM.[2]
A phase I clinical study showed Daclatasvir's inhibition for HCV viruses. A 1 mg dose of daclatasvir produced a mean 1.8 log10 reduction in serum HCV RNA 24 h after administration. The 10 and 100 mg doses produced 3.2 log10 and 3.3 log10 reductions, respectively. Data collected from clinical trials on daclatasvir illustrated an initial, rapid viral decline followed by a slower fall in HCV RNA, which indicated that by inhibiting NS5A, daclatasvir blocks intracellular HCV RNA synthesis and virion assembly and secretion.[1]
References:
1.Herbst D A, Reddy K R. NS5A inhibitor, daclatasvir, for the treatment of chronic hepatitis C virus infection[J]. Expert opinion on investigational drugs, 2013, 22(10): 1337-1346.
2.Gao M, Nettles R E, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect[J]. Nature, 2010, 465(7294): 96-100.
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















