Fevipiprant (Synonyms: NVP-QAW039, QAW039) |
| Catalog No.GC19153 |
Fevipiprant (QAW039, NVP-QAW039) est un antagoniste des récepteurs de la prostaglandine D2 (DP2) sélectif et réversible, actif par voie orale, avec une valeur Kd de 1,14 nM.
Products are for research use only. Not for human use. We do not sell to patients.
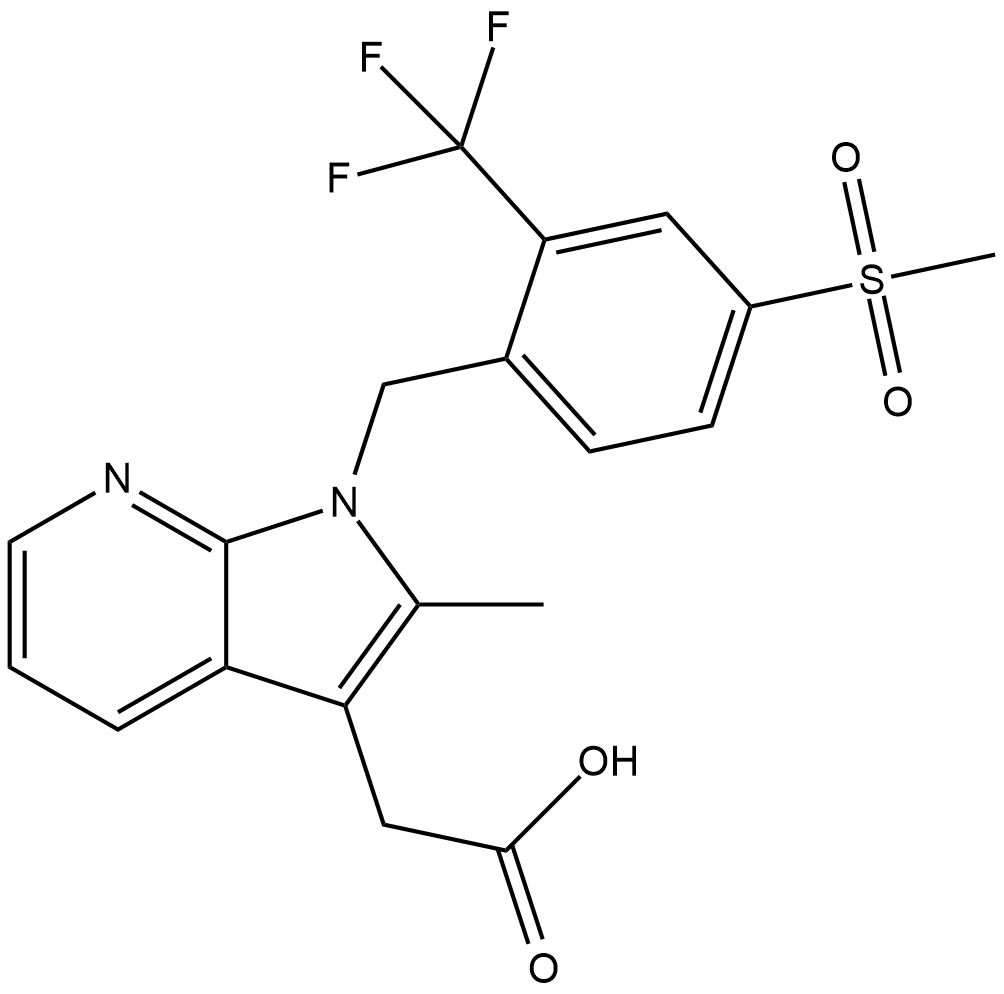
Cas No.: 872365-14-5
Sample solution is provided at 25 µL, 10mM.
Fevipiprant(QAW039) is a selective, potent, reversible competitive CRTh2 antagonist with an in vitro dissociation constant KD value of 1.1nM at the CRTh2 receptor and an IC50 value of 0.44 nM for inhibition of PGD2-induced eosinophil shape change in human whole blood.IC50:0.44 nM(PGD2-induced eosinophil shape change)Kd value:1.1nM(CRTh2 receptor)[1]In vitro: CRTh2-mediated shape change in eosinophils was used to profile QAW039 in whole blood and represents a physiologically relevant environment. The comparable IC50 values for QAW039 in the whole blood and isolated shape-change assays are consistent with its lower plasma-protein binding and its relatively slow dissociation kinetics that drive its increased potency .QAW039 is highly potent in whole-blood systems, with the IC50 value obtained consistent with the affinity values calculated from radioligand experiments. In a further disease-relevant cellular context, the potency of QAW039 in the isolated Th2 cell cytokine inhibition assay is consistent with its CRTh2 receptor affinity, and, as with eosinophil assay readouts, this represents an improved potency compared with QAV680[2].
References:
[1]. Erpenbeck, V. J. et al. Pharmacokinetics, Safety, and Tolerability of Fevipiprant (QAW039), a Novel CRTh2 Receptor Antagonist: Results From 2 Randomized, Phase 1, Placebo-Controlled Studies in Healthy Volunteers. Clinical pharmacology in drug development 5, 306-313, doi:10.1002/cpdd.244 (2016).
[2]. Sykes, D. A. et al.Fevipiprant (QAW039), a Slowly Dissociating CRTh2 Antagonist with the Potential for Improved Clinical Efficacy. Molecular pharmacology 89, 593-605, doi:10.1124/mol.115.101832 (2016).
Average Rating: 5 (Based on Reviews and 28 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















